Question: Redo Problem 13.25 using Aspen Plus. Problem 13.25 When pure hydrogen iodide gas enters an evacuated cylinder, the following reactions may occur: (Note that since
Redo Problem 13.25 using Aspen Plus.
Problem 13.25
When pure hydrogen iodide gas enters an evacuated cylinder, the following reactions may occur:
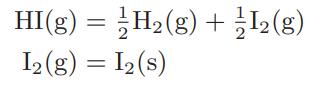
(Note that since Gibbs energies of formation data are available for iodine in both the gaseous and solid phases, it is more convenient to think of the solid-vapor iodine phase equilibrium as a chemical equilibrium.) If the reaction mixture is gradually compressed at 25°C, a pressure is reached at which the first bit of solid iodine appears. What is the pressure at which this occurs, and what is the vapor composition at this pressure?
(s) I = (8) T (8) + (8)H = (8)IH
Step by Step Solution
3.51 Rating (154 Votes )
There are 3 Steps involved in it
To model this problem in Aspen Plusfollow these steps Create a new Aspen Plus model and add the foll... View full answer

Get step-by-step solutions from verified subject matter experts


