Question: Redo Problem 13.3 using Aspen Plus. Problem 13.3 Carbon dioxide can react with graphite to form carbon monoxide, and the carbon monoxide formed can further
Redo Problem 13.3 using Aspen Plus.
Problem 13.3
Carbon dioxide can react with graphite to form carbon monoxide,
![]()
and the carbon monoxide formed can further react to form carbon and oxygen:
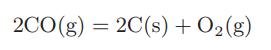
Determine the equilibrium composition when pure carbon dioxide is passed over a hot carbon bed maintained at 1 bar and
(a) 2000 K or
(b) 1000 K.
C(graphite) + CO2(g) = 2CO(g)
Step by Step Solution
3.49 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


