Question: Redo Problem 13.1 using Aspen Plus. Problem 13.1 Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction
Redo Problem 13.1 using Aspen Plus.
Problem 13.1
Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction
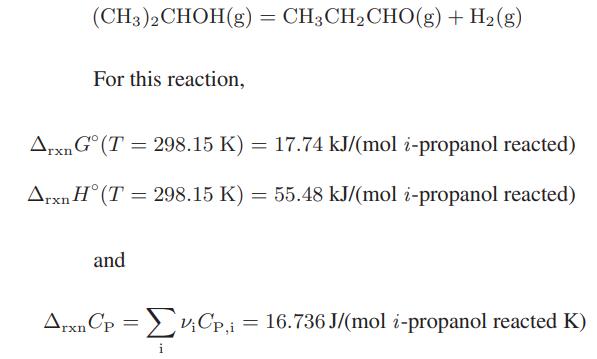
Compute the equilibrium fraction of isopropyl alcohol that would be dehydrogenated at 500 K and 1.013 bar.
(CH3)2CHOH(g) = CH3CHCHO(g) + H(g) For this reaction, Arxn G (T= 298.15 K) = 17.74 kJ/(mol i-propanol reacted) Arxn H (T = 298.15 K) = 55.48 kJ/(mol i-propanol reacted) and ArxnCp = VCp,i = 16.736 J/(mol i-propanol reacted K) vCp, i
Step by Step Solution
★★★★★
3.30 Rating (153 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


