Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction
Question:
Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction
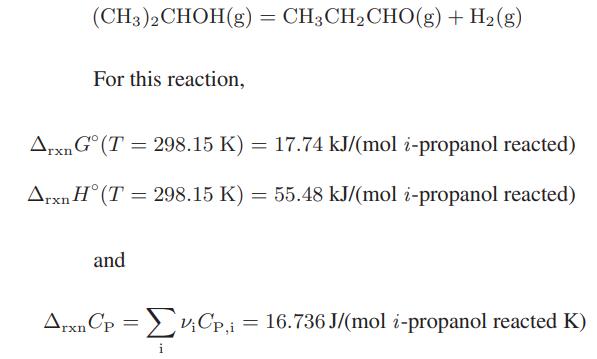
Compute the equilibrium fraction of isopropyl alcohol that would be dehydrogenated at 500 K and 1.013 bar.
Transcribed Image Text:
(CH3)2CHOH(g) = CH3 CH₂CHO(g) + H₂(g) For this reaction, Arxn Gᵒ (T = 298.15 K) = 17.74 kJ/(mol i-propanol reacted) Arxn H (T = 298.15 K) = 55.48 kJ/(mol i-propanol reacted) and ArxnCp = V₁Cp,i = 16.736 J/(mol i-propanol reacted K) ΣvCp, i
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
The information you sent shows the following reaction CH32CHOHg CH3CH2CHOg H2g This reaction is dehy...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Redo Problem 13.1 using Aspen Plus. Problem 13.1 Isopropyl alcohol is to be dehydrogenated in the gas phase to form propionaldehyde according to the reaction Compute the equilibrium fraction of...
-
An electroplating process supplies a coating thickness of 800 nm. Calculate the area which would be covered using 1 kg of gold, mindful that the density of gold is 19300 kg/m3
-
The decomposition of iodoethane in the gas phase proceeds according to the following equation: C2H5I(g) C2H4(g) + HI(g) At 660. K, k = 7.2 10-4 s-1; at 720. K, k = 1.7 10-2 s-1. What is the rate...
-
In Exercises, find the limit. x-4 lim x-00x + 1
-
John Biggs and Patty Jorgenson are both cost accounting managers for a manufacturing division. During lunch yesterday, Patty told John that she was planning on quitting her job in three months...
-
Consider the first-order RC circuit shown in Figure 6.24. a. Derive the input-output differential equation relating \(v_{C}\) and \(v_{\mathrm{a}}\). b. Determine the transfer function \(I(s) /...
-
Why are capital budgeting decisions so important? AppendixLO1
-
Poco Company purchased 85% of the outstanding common stock of Serena Company on December 31, 2009, for $310,000 cash. On that date, Serena Companys stockholders equity consisted of the following:...
-
Chiptech, Inc., is an established computer chip firm with several profitable existing products as well as some promising new products in development. The company earned $1.70 a share last year, and...
-
Ionic liquids are salts with melting temperatures that are sufficiently low that they are liquids at or near room temperature. They consist of a larger cation and a smaller anion, for example,...
-
Carbon dioxide can react with graphite to form carbon monoxide, and the carbon monoxide formed can further react to form carbon and oxygen: Determine the equilibrium composition when pure carbon...
-
From tables provided in this chapter, approximate the full load rating for a 112 hp, 120 V, single-phase electric motor, in amperes. TABLE 18.24 APPROXIMATE FULL LOAD RATING FOR SELECTED MOTORS, IN...
-
1) Explain the following paragraph in your own words. "A nation which has can produce at a lower cost when measured in terms of opportunity cost is said to have a comparative advantage. Even though...
-
3. Two companies (A and B) are duopolists that produce identical products. Demand for the products is given by the following demand function: P = 10,000 QA- QB - where QA and QB are the quantities...
-
Consider the following initial-value problem. f'(x) = 2ex - 6x; f(0) = 4 Integrate the function f'(x). (Remember the constant of integration.) || | f'(x)dx = Find the value of C using the condition...
-
The value chain is based on primary activities logstica Operations External logistics Marketing and sales Service and are complemented by support activities Company infrastructure is what it is,...
-
On average, both arms and hands together account for 13% of a person's mass, while the head is 7.0% and the trunk and legs account for 80%. We can model a spinning skater with her arms outstretched...
-
Discuss the advantages and disadvantages of using sampling to reduce the number of data objects that need to be displayed. Would simple random sampling (without replacement) be a good approach to...
-
Describe a group you belong or have belonged discuss the stages of group development and suggest how to improve the group effectiveness by using the group development model.
-
Determine the moment about point A of each of the three forces acting on the beam. F = 375 lb F = 500 lb . 0.5 ft 8 ft 6 ft 5ft- 5 ft- 30 F3 = 160 lb
-
Determine the moment about point B of each of the three forces acting on the beam. F = 375 lb F2 = 500 lb 3. B. 0.5 ft tontsa- 8 ft 6 ft -5 ft- 30 F3 = 160 lb
-
The crowbar is subjected to a vertical force of P = 25 lb at the grip, whereas it takes a force of F = 155 lb at the claw to pull the nail out. Find the moment of each force about point A and...
-
whether the following statements is TRUE or FALSE by providing a brief explanation . b) The Value at Risk of a first project is 8 and the Value at Risk of a second project is 5. The Value at Risk of...
-
Fill out a spreadsheet using the following information to perform a NPV analysis. Revenues in each of years 1-3 = $20,000 Year 0 initial investment = $40,000 Inventory level = $10,000 in year 1,...
-
A firm has $9.5 Billion debt outstanding, with a yield to maturity of 5.3% and a coupon rate of 4.6%. They have 148 million preferred shares outstanding, currently trading at $92.29. They also have...

Study smarter with the SolutionInn App


