Question: Repeat Problem 3.25, now considering nitrogen to be a real gas with the thermodynamic properties given in Fig. 3.3-3. Problem 3.25 A 0.01-m 3 cylinder
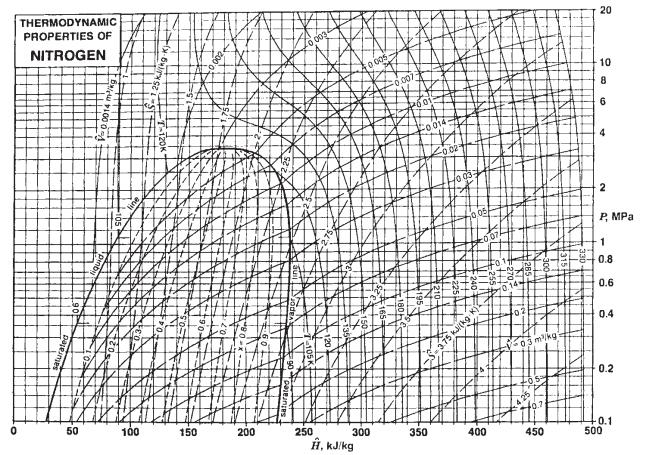
Repeat Problem 3.25, now considering nitrogen to be a real gas with the thermodynamic properties given in Fig. 3.3-3.
Problem 3.25
A 0.01-m3 cylinder containing nitrogen gas initially at a pressure of 200 bar and 250 K is connected to another cylinder 0.005 m3 in volume, which is initially evacuated. A valve between the two cylinders is opened until the pressures in the cylinders equalize. Find the final temperature and pressure in each cylinder if there is no heat flow into or out of the cylinder. You may assume that there is no heat transfer between the gas and the cylinder walls and that the gas is ideal with a constantpressure heat capacity of 30 J/(mol K).
Fig. 3.3-3.
0 THERMODYNAMICA A PROPERTIES OF NITROGEN 50 P1000 100 150 200 0 0034 250 0.005 300 . kJ/kg -0.007 350 014 2002 to 03- 50.05 20.077 S-3.75 kaitkg Ki 400 0.14 02 03 mkg? 450 20 10 8 6 A 2 P, MPa 1 C 0.8 0.6 0.4 0.2 +0.1 500
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


