Show that for mixing of ideal gases at constant temperature and pressure to form an ideal gas
Question:
Show that for mixing of ideal gases at constant temperature and pressure to form an ideal gas mixture,
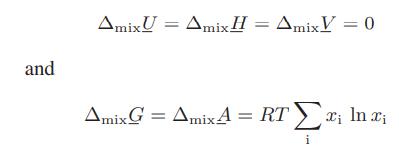
Transcribed Image Text:
and SmixU = SmixH SmixH = ∆mixV = 0 SmixG = SmixA = RT Σ; Invi
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To show that for mixing of ideal gases at constant temperature and pressure to form an ideal gas mixturethe internal energy changeenthalpy changevolum...View the full answer

Answered By

Deborah Joseph
My experience has a tutor has helped me with learning and relearning. You learn everyday actually and there are changes that are made to the curriculum every time so being a tutor has helped in keeping me updated about the present curriculum and all.
I have also been able to help over 100 students achieve better grades particularly in the categories of Math and Biology both in their internal examinations and external examinations.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
In Sec. 9.1 we considered the changes in thermodynamic properties on forming an ideal gas mixture from a collection of ideal gases at the same temperature and pressure. A second, less common way of...
-
What are the primary data sources for tracking youthful offender crime nationwide? What are the methodological strengths and limitations of these sources?
-
Two mass streams of two different ideal gases are mixed in a steady-flow chamber while receiving energy by heat transfer from the surroundings. The mixing process takes place at constant pressure...
-
Suppose that there is parity between the Australian dollar and the United States dollar. Let x be a positive number and assume that the United States dollar increases by 100x % in Australian dollars....
-
The following balance sheet was prepared by the accountant for Tippetts Company. Instructions: Prepare a corrected classified balance sheet using appropriate accounttitles. Tippetts Company Balance...
-
Aggregating across products, retailers, or suppliers in a single order allows for an increase in lot size for individual products. an increase in customer demand. a reduction in holding cost per...
-
Why has trade union membership remained so high in the public sector when it has declined so markedly in the private sector? LO3
-
The Podrasky Corporation is considering a $200 million expansion (capital expenditure) program next year. The company wants to know approximately how much additional financing (if any) will be...
-
Download and save the photos to view them Download and save the photos to view them Download and save the photos to view them Download and save the photos to view them Sales Accounts Consolidation...
-
In each case, write a program implemented using Spark (either on AWS or Databricks), to: Find the 5 most frequent and 5 least frequent (but present)t bi-grams for your dataset (only digits, not the...
-
Assuming that two pure fluids and their mixture can be described by the van der Waals equation of state, and that for the mixture the van der Waals one-fluid mixing rules apply a. Show that the...
-
The volume change on mixing in cm 3 /mol for ethanol(1) + methyl butyl ether(2) mixtures at 25C is given by the following equation Given that V 1 = 58.63 cm 3 /mol and V 2 = 118.46 cm 3 /mol,...
-
(Appendix) Which of the following is true concerning significantly large labor variances? a. They are prorated among Work in Process, Finished Goods, and Cost of Goods Sold. b. They are closed to...
-
The water level in a tank is \(20 \mathrm{~m}\) above the ground. A hose is connected to the bottom of the tank, and the nozzle at the end of the hose is pointed straight up. The tank cover is...
-
A simple experiment has long been used to demonstrate how negative pressure prevents water from being spilled out of an inverted glass. A glass that is fully filled by water and covered with a thin...
-
A golf ball is hit on a level fairway. When it lands, its velocity vector has rotated through an angle of 90. What was the launch angle of the golf ball? Pyo By Dyz =0 Uso Range R x max dya
-
Repeat Prob. 10-18 for signed-magnitude binary numbers. Prob. 10-18 Derive an algorithm in flowchart form for the comparison of two signed binary numbers when negative numbers are in signed-2's...
-
Tideview Home Health Care, Inc., has a bond issue outstanding with eight years remaining to maturity, a coupon rate of 10 percent with interest paid annually, and a par value of $1,000. The current...
-
a. Based on a review of the characteristics of Fig. 5.126, which parameter changed the most with increase in temperature? b. Which changed the least? c. What are the maximum and minimum values of...
-
Let (x) = x 2 - 9, g(x) = 2x, and h(x) = x - 3. Find each of the following. (((--) 2
-
List three products that can be used equally well by people with and without visual impairments and explain why.
-
Imagine you are tasked with designing a single dishwasher for both the European and American markets. Determine a set of global, social, environmental, and economic issues you would have to consider...
-
Find a product specification sheet for a consumer product such as an automobile, appliance, TV, motor, or something similar, and determine whether the specifications are easy to interpret. For...
-
In 1975 the price of a new house was $48,273. In 2020 the price of a new house is $185,524. How much has the price of housing increased over the entire time period in percentage terms? State your...
-
CHOP Inc., which makes only one product, Yester, has the following information available for the coming year. CHOP expects sales to be 27,000 units at $32 per unit. The current inventory of Yester is...
-
Huron Company produces a commercial cleaning compound known as Zoom. The direct materials and direct labor standards for one unit of Zoom are given below: Standard Quantity or Standard Hours Standard...

Study smarter with the SolutionInn App


