Values of the virial coefficients B and C at a fixed temperature can be obtained from experimental
Question:
Values of the virial coefficients B and C at a fixed temperature can be obtained from experimental PV T data by noting that
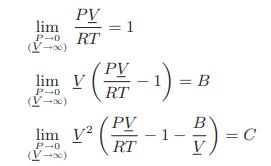
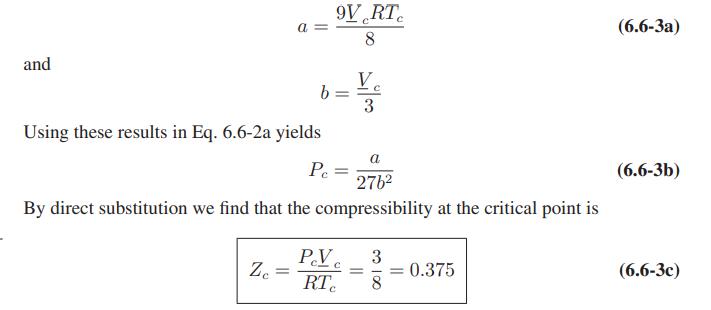
a. Using these formulas, show that the van der Waals equation leads to the following expressions for the virial coefficients.
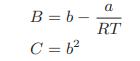
b. The temperature at which
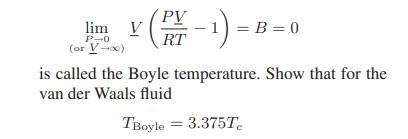
where Tc is the critical temperature of the van der Waals fluid given by Eqs. 6.6-3. (For many real gases TBoyle is approximately 2.5Tc!)
Eqs. 6.6-3.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





