A (50.0 mathrm{~cm}) long column contains activated carbon and is initially saturated with acetic acid at (mathrm{c}=0.007
Question:
A \(50.0 \mathrm{~cm}\) long column contains activated carbon and is initially saturated with acetic acid at \(\mathrm{c}=0.007 \mathrm{kmol} / \mathrm{m}^{3}\) at \(4^{\circ} \mathrm{C}\). At \(\mathrm{t}=0\), column is eluted with pure water \((\mathrm{C}=0)\) at \(60^{\circ} \mathrm{C}\) at a superficial velocity of \(12.0 \mathrm{~cm} / \mathrm{min}\). Data are in problem 20.D7.
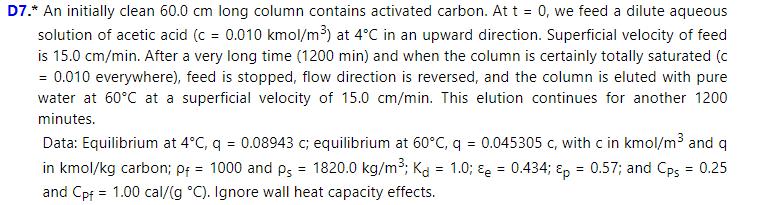
a. At what time does temperature increase?
b. What does the outlet concentration profile look like? Give concentration values and times for changes in exiting fluid.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





