Question: A flash drum operating at (300 mathrm{kPa}) is separating (1000.0 mathrm{kmol} / mathrm{h}) of a mixture that is (40.0 mathrm{~mol} %) isobutane, (25.0 % mathrm{n})-pentane,
A flash drum operating at \(300 \mathrm{kPa}\) is separating \(1000.0 \mathrm{kmol} / \mathrm{h}\) of a mixture that is \(40.0 \mathrm{~mol} \%\) isobutane, \(25.0 \% \mathrm{n}\)-pentane, and \(35.0 \% \mathrm{n}\)-hexane. We wish a \(90.0 \%\) recovery of \(n\)-hexane in the liquid. Find \(T_{\text {drum }}, x_{i}, y_{i}\), and V/F. Use DePriester charts or Eq. (2-28). \(90.0 \%\) recovery of \(n\)-hexane in the liquid. Find \(T_{\text {drum }}, x_{i}, y_{i}\), and V/F. Use DePriester charts or Eq. (2-28).
Equations (2-28)
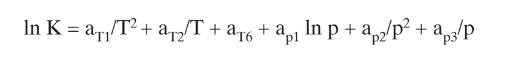
In K = a/T2+ a2 T + + pl In p + ap2/p + ap3/p
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


