A mixture of (beta)-methylnaphthalene (+beta)-chloronaphthalene ((beta mathrm{cn})) at (1.0 mathrm{~atm}) (Figure 17 (8 mathrm{~A})) has liquid and
Question:
A mixture of \(\beta\)-methylnaphthalene \(+\beta\)-chloronaphthalene \((\beta \mathrm{cn})\) at \(1.0 \mathrm{~atm}\) (Figure 17 \(8 \mathrm{~A})\) has liquid and solid in equilibrium at \(50^{\circ} \mathrm{C}\).
Figure (17-8A)
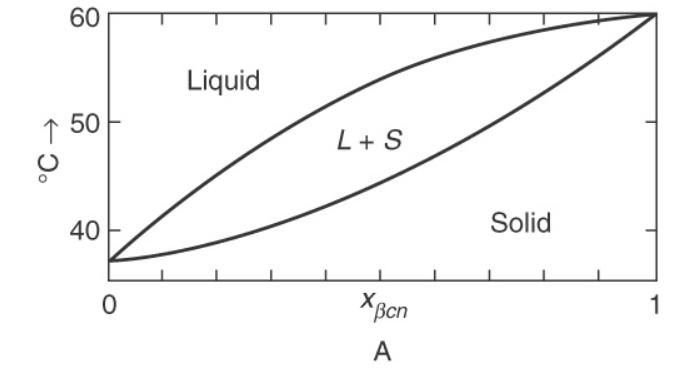
a. What are the mole fractions \(\left(\mathrm{x} \beta_{\mathrm{cn}}\right)\) of the liquid and solid phases in equilibrium?
b. What ranges of initial mole fraction can produce an equilibrium mixture at \(50^{\circ} \mathrm{C}\) ?
c. If the initial value of \(x \beta_{\mathrm{cn}}=0.5\) and the liquid and solid are in equilibrium at \(50^{\circ} \mathrm{C}\), what fraction of the original feed is liquid?
d. If the liquid in parts a and \(\mathrm{c}\) is removed and then cooled to \(45^{\circ} \mathrm{C}\), what are the mole fractions of the liquid and solid in equilibrium?
e. If the liquid in parts a and \(\mathrm{c}\) is removed and then cooled to \(35^{\circ} \mathrm{C}\), what is the average mole fraction of the solid?
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





