In crystallization of potassium chloride with a (100^{circ} mathrm{C}) feed and the crystallizer operating at (20^{circ} mathrm{C}),
Question:
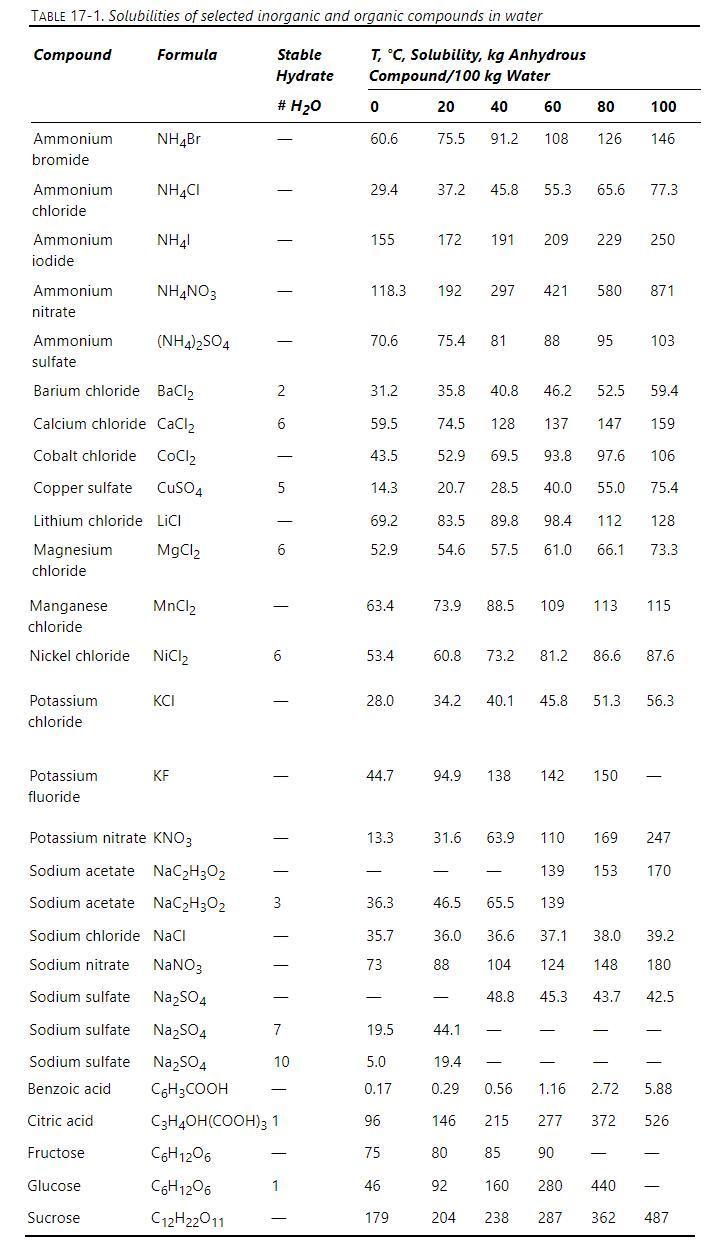
In crystallization of potassium chloride with a \(100^{\circ} \mathrm{C}\) feed and the crystallizer operating at \(20^{\circ} \mathrm{C}\), we obtain \(\mathrm{G}=4.0 \times 10^{-7} \mathrm{~m} / \mathrm{s}\). The crystallizer's volume is \(0.2 \mathrm{~m}^{3}\), and the feed rate is \(1000 \mathrm{~kg} / \mathrm{h}\). When operated without seeds, the crystals produced are too small. Your boss decides to seed and use a new crystallizer with volume \(=1.0 \mathrm{~m}^{3}\) but with the same throughput of feed and the same supersaturation, \(\mathrm{C}-\mathrm{C}^{*}\), as the current crystallizer. Desired crystal size is \(2.56 \mathrm{~mm}\). If the seed crystals are available with a very tight size distribution, what size of seed crystals, \(\mathrm{L}_{\mathrm{S}}(\mathrm{mm})\), and what weight of seed crystals \((\mathrm{kg} / \mathrm{h})\) should be used?
Data: \(ho_{\text {feed }}=1209 \mathrm{~kg} / \mathrm{m}^{3}\). Crystals are cubic in shape with equal growth on all faces, and \(\mathrm{k}_{\mathrm{v}}\) \(=1.0\). Solubility and hydrate data are in Table 17-1. Note: Watch your units!
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





