Question:
Nickel chloride solubility data are given in Table \(17-1\). We start with \(100 \mathrm{~kg}\) of water saturated with \(\mathrm{NiCl}_{2}\) at \(100^{\circ} \mathrm{C}\) and cool to \(10^{\circ} \mathrm{C}\).
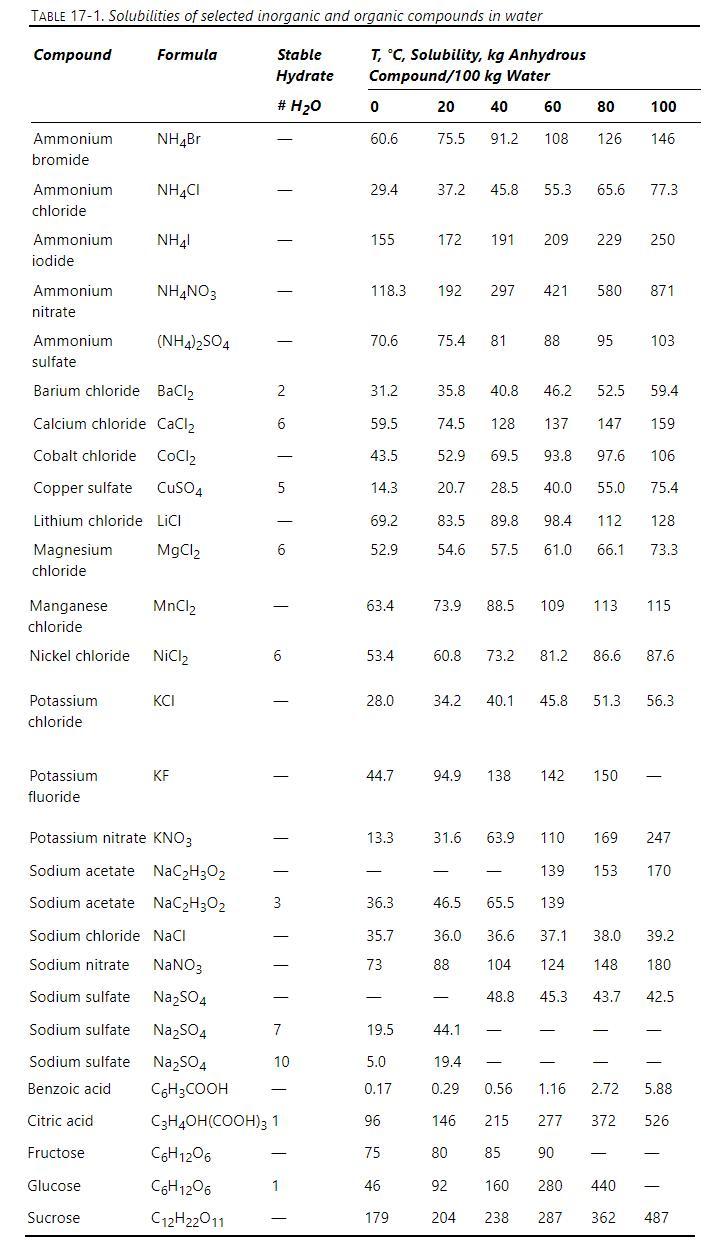
a. What is the solubility of anhydrous \(\mathrm{NiCl}_{2}\) at \(100^{\circ} \mathrm{C}\) ?
b. What is the solubility of anhydrous \(\mathrm{NiCl}_{2}\) at \(10^{\circ} \mathrm{C}\) ?
c. At \(10^{\circ} \mathrm{C}\), how many \(\mathrm{kg}\) of anhydrous \(\mathrm{NiCl}_{2}\) precipitate, how many \(\mathrm{kg}\) of \(\mathrm{NiCl}_{2}\) hydrate precipitate, how many \(\mathrm{kg}\) of water of hydration precipitate, and how many \(\mathrm{kg}\) of water remain in solution?
d. If we want to produce the initial feed by dissolving hydrate crystals in water at \(100^{\circ} \mathrm{C}\), how many \(\mathrm{kg}\) of hydrate crystals would you dissolve in how much water?
Transcribed Image Text:
TABLE 17-1. Solubilities of selected inorganic and organic compounds in water Compound Formula Stable Hydrate T, C, Solubility, kg Anhydrous Compound/100 kg Water # HO 0 20 40 60 80 100 Ammonium NH4Br 60.6 75.5 91.2 108 126 146 bromide Ammonium NH4Cl 29.4 37.2 45.8 55.3 65.6 77.3 chloride Ammonium NH 155 172 191 209 229 250 iodide Ammonium NH4NO 118.3 192 297 421 580 871 nitrate Ammonium (NH4)2SO4 70.6 75.4 81 88 95 103 sulfate Barium chloride BaCl2 Calcium chloride CaCl2 Cobalt chloride CoCl2 Copper sulfate CuSO4 Lithium chloride LiCI 26 56 31.2 35.8 40.8 46.2 52.5 59.4 59.5 74.5 128 137 147 159 43.5 52.9 69.5 93.8 97.6 106 14.3 20.7 28.5 40.0 55.0 75.4 69.2 83.5 89.8 98.4 112 128 52.9 54.6 57.5 61.0 66.1 73.3 Magnesium MgCl2 chloride Manganese MnCl2 63.4 73.9 88.5 109 113 115 chloride Nickel chloride NiCl2 6 53.4 60.8 73.2 81.2 86.6 87.6 Potassium KCI 28.0 34.2 40.1 45.8 51.3 56.3 chloride Potassium fluoride KF FF 44.7 94.9 138 142 150 Potassium nitrate KNO3 Sodium acetate NaC2H3O2 Sodium acetate NaC2H3O2 3 Sodium chloride NaCl Sodium nitrate NaNO3 73 Sodium sulfate Na2SO4 Citric acid Sodium sulfate Na2SO4 7 19.5 44.1 Sodium sulfate Na2SO4 10 5.0 19.4 Benzoic acid C6H3COOH C3H4OH(COOH); 1 - Fructose C6H12O6 - Glucose Cotiz 1 46 Sucrose C12H22O11 | | 8 p 13.3 31.6 63.9 110 169 247 139 153 170 36.3 46.5 65.5 139 35.7 36.0 36.6 37.1 38.0 39.2 88 104 124 148 180 48.8 45.3 43.7 42.5 0.17 0.29 0.56 1.16 2.72 5.88 146 215 277 372 526 80 85 90 - 179 92 160 280 440 179 204 238 287 362 487







