Produce pure water from seawater by boiling it with n-decane vapor (see figure). This is sort of
Question:
Produce pure water from seawater by boiling it with n-decane vapor (see figure). This is sort of the reverse of steam distillation. Seawater is roughly \(3.5 \mathrm{wt} \%\) salt, which can be approximated as \(\mathrm{NaCl}\). The feed is \(1000.0 \mathrm{~kg} / \mathrm{h}\) seawater at \(30.0^{\circ} \mathrm{C}\). The feed is heated with pure saturated n-decane vapor at \(760.0 \mathrm{~mm} \mathrm{Hg}\). Most of the n-decane vapor condenses while the remainder is carried overhead with the water vapor. Pressure is \(760.0 \mathrm{~mm}\) \(\mathrm{Hg}\). Recover \(60.0 \%\) of the water as condensate.
a. Find the approximate still temperature.
b. Find the \(\mathrm{mol} / \mathrm{h}\) of \(\mathrm{n}\)-decane carried over in the vapor.
c. What is the weight fraction of salt in the waste stream? Disposing of waste from desalination plants that are not next to the ocean is a major problem.
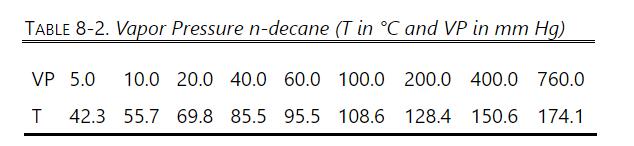
Data: VP of n-decane: Table 8-2, VP of water: Table 8-1, \(\mathrm{MW}_{\text {water }}=\) 18.016, \(\mathrm{MW}_{\mathrm{NaCl}}=58.45, \mathrm{MW}_{\mathrm{C} 10}=142.28\), salt is nonvolatile. Water and n-decane are immiscible. \(\mathrm{NaCl}\) dissolves only in water.

Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





