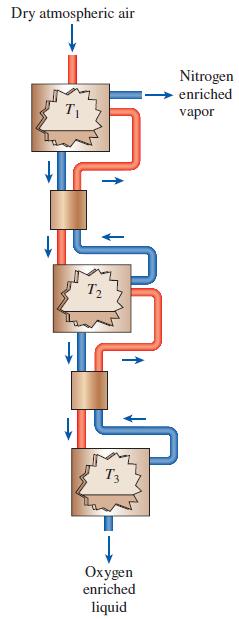
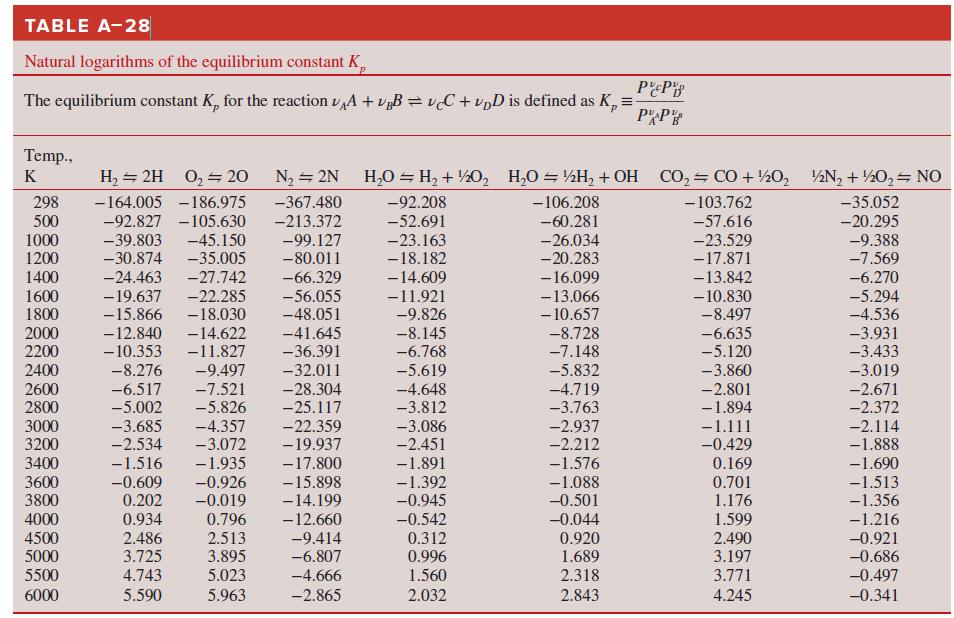
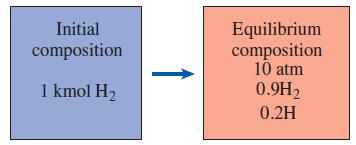
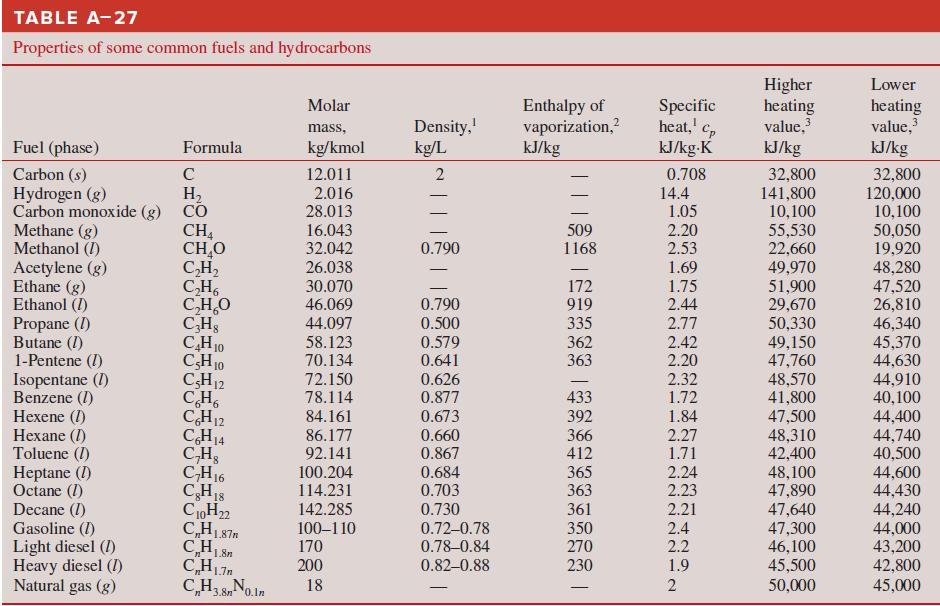
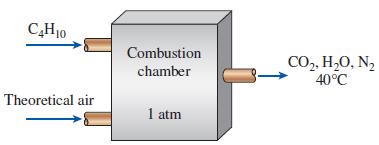
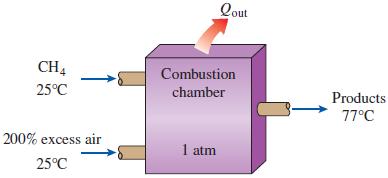
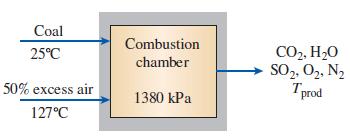
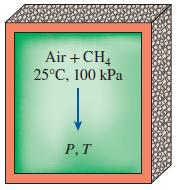
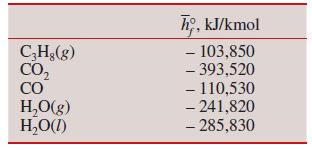
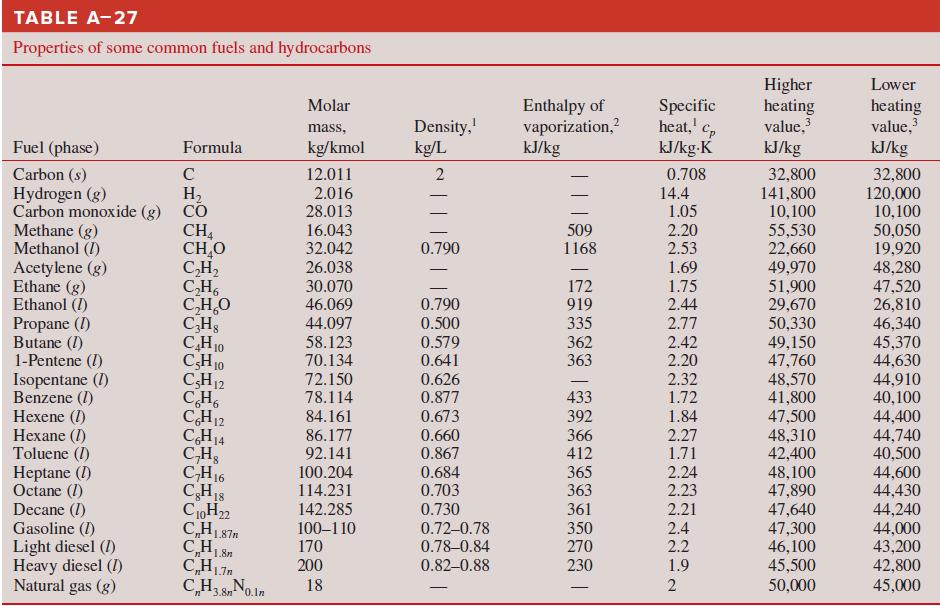
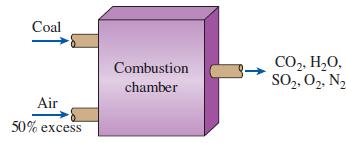
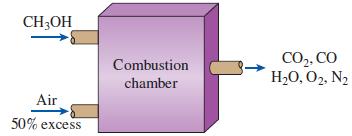
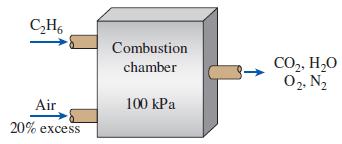
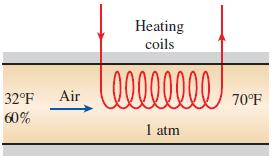
Thermodynamics An Engineering Approach 9th Edition Yunus Cengel, Michael Boles, Mehmet Kanoglu - Solutions
Looking for comprehensive insights into "Thermodynamics An Engineering Approach 9th Edition" by Yunus Cengel, Michael Boles, and Mehmet Kanoglu? Our platform offers an extensive collection of questions and meticulously solved problems from the textbook, ensuring that you have the best resources at your fingertips. With our solutions manual and chapter solutions, you'll find detailed step-by-step answers and an instructor manual to guide your learning experience. Explore the solved problems and test bank, all available for free download, to deepen your understanding and ace your exams. Access our online answers key and solutions PDF for an unbeatable learning journey.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()