Reconsider Prob. 1588. The automobile engine is to be converted to natural gas (methane, CH 4 )
Question:
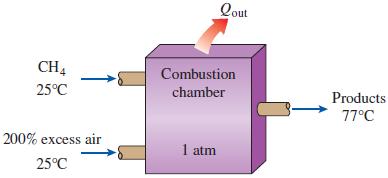
Reconsider Prob. 15–88. The automobile engine is to be converted to natural gas (methane, CH4) fuel. Assuming that all factors remain the same, what is the maximum work that can be produced by the modified engine, in kJ/kg fuel?
Data From Q#88:
n-Octane [C8H18(l)] is burned in an automobile engine with 200 percent excess air. Air enters this engine at 1 atm and 25°C. Liquid fuel at 25°C is mixed with this air before combustion. The exhaust products leave the exhaust system at 1 atm and 77°C. What is the maximum amount of work, in kJ/kg fuel, that can be produced by this engine? Take T0 = 25°C.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:





