Use the Gibbs function to determine the equilibrium constant of the H 2 O H 2
Question:
Use the Gibbs function to determine the equilibrium constant of the H2O ⇌ H2 + 1/2 O2 reaction at
(a) 1440 R
(b) 3960 R.
How do these compare to the equilibrium constants of Table A–28?
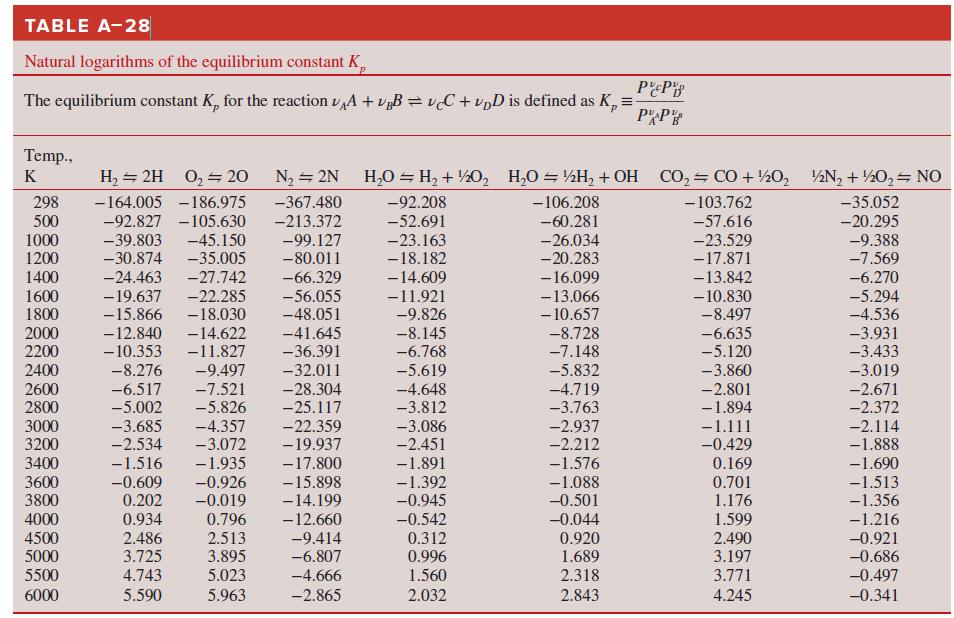
Transcribed Image Text:
TABLE A-28 Natural logarithms of the equilibrium constant K, The equilibrium constant K, for the reaction vA + v„B = vC + vpD is defined as K, = PP Temp., K H, = 2H 0, 20 N2 = 2N H,0 = H, + ½0, H,O = ½H, + OH CO, CO + ½0, ½N, + ½0, NO 298 500 1000 -164.005 -186.975 -367.480 -92.208 -106.208 - 103.762 -35.052 -92.827 -105.630 -45.150 -35.005 -27.742 -213.372 -52.691 -60.281 -57.616 -20.295 -39.803 -30.874 -99.127 -80.011 -9.388 -7.569 -23.163 -26.034 -20.283 -23.529 -17.871 -13.842 1200 -18.182 -6.270 -5.294 1400 -24.463 -66.329 -14.609 1600 1800 -19.637 -15.866 -22.285 -18.030 -14.622 - 16.099 -13.066 - 10.657 -56.055 -48.051 -11.921 -9.826 - 10.830 -8.497 -6.635 -5.120 -4.536 2000 2200 -12.840 - 10.353 -41.645 -8.145 -6.768 -8.728 -7.148 -3.931 -3.433 -11.827 -36.391 2400 -8.276 -9.497 -32.011 -5.619 -4.648 -3.812 -5.832 -3.860 -3.019 -28.304 2600 2800 -6.517 -5.002 -7.521 -5.826 -4.719 -3.763 -2.801 -1.894 -2.671 -2.372 -25.117 -22.359 3000 3200 -3.685 -2.534 -4.357 -3.072 -3.086 -2.937 -2.212 -1.111 -0.429 -2.114 -1.888 -19.937 -2.451 3400 -1.516 -1.935 -17.800 -1.891 -1.576 0.169 3600 3800 -0.609 0.202 -0.926 -0.019 -15.898 -14.199 -12.660 -1.690 -1.513 -1.356 -1.392 -0.945 -1.088 -0.501 0.701 1.176 0.796 2.513 3.895 0.934 -0.542 -0.044 0.920 1.689 4000 1.599 -1.216 4500 5000 2.486 3.725 -9.414 -6.807 0.312 2.490 3.197 -0.921 -0.686 0.996 5500 4.743 5.023 -4.666 1.560 2.318 3.771 -0.497 6000 5.590 5.963 -2.865 2.032 2.843 4.245 -0.341
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
The equilibrium constant of the reaction is to be dete...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
The equilibrium constant for the reaction H2 + at 1 atm and 1500C is given to be K. Of the reactions given below, all at 1500C, the reaction that has a different equilibrium constant is (a) H2 + 12O2...
-
The equilibrium constant for the H2 + ½ O2 H2O reaction at 1 atm and 1200 K is KP. Use this information to determine the equilibrium constant for the following reactions: (a) at l atm H, +...
-
The table below shows data (from a 2004 Bureau of the Census report) on the number of times 20- to 24-year-old men have been married. a. Verify that the mean number of times men have been married is...
-
Consider a portfolio of the following derivatives where the counterparty is an OECD bank. Derivative 8-year interest rate swap 6-month option on an equity 1-year swap on precious metals 9-month...
-
Digits (0, 1, 2,. . . ,9) are randomly selected for telephone numbers in surveys. The random variable x is the selected digit. a. Find the mean and standard deviation of x. b. Find the z score for...
-
Asitsyear-endapproaches,itappearsthatMendezCorporation'snetincomewillincrease10%thisyear.ThepresidentofMendezCorporation,nervousthatthestockholdersmightexpectthecompanytosustainthis10%growthrateinneti...
-
4 Desde hace aos, Ferrari tiene fama de ser fabricante de automviles de lujo muy costosos. Esta empresa planifica atraer al principal segmento del mercado de compra de automviles, cuyo precio se...
-
We would like to execute the loop below as efficiently as possible. We have two different machines, a MIMD machine and a SIMD machine. for (i=0; i < 2000; i++) for (j=0; j <3000; j++) X_array[i][j] =...
-
Analyzer x C Ratio of x G Ratio of Lix C Recent Bal x C Search Tex Cengage gament takeAssignment Main.do?invokeretakeAssignmentSessionLocator&inprogress-false Show Me How Calculator The following...
-
a. Discuss the various operational budgets prepared for: (i) a service organisation (ii) a trading organisation. b. (i) How do firms handle the complexity of calculating breakeven when there are many...
-
A mixture of ideal gases consists of the following gases by mole fraction: 10 percent CO 2 , 60 percent H 2 O, and 30 percent CO. Determine the Gibbs function of the CO in this mixture when the...
-
An inventor claims she can produce hydrogen gas by the reversible reaction 2H 2 O H 2 + O 2 . Determine the mole fractions of the hydrogen and oxygen produced when this reaction occurs at 4000 K and...
-
Appliance Sales Corporation sells all types of appliances. In addition, it offers purchasers of its appliances the option of purchasing repair contracts. During the current year, Appliance estimates...
-
What are factors which hamper the promotion of an entrepreneurial culture in South Africa?
-
Please Help P/R End Date 2/8/2019 Company Name: Prevosti Farms and Sugarhouse Check Date 2/13/2019 Tax Name M/S # of W/H Hourly Rate or Period # of Regular # of Overtime # of Holiday Wage Hours Hours...
-
Read the description of following adjustments that are required at the end of the accounting period for Hubbard Repair Services, a new firm. Determine the account and amount to be debited and the...
-
Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that time has just flown by; over twenty-four years...
-
Here are summary statistics for randomly selected weights of newborn girls: n = 36, x = 3180.6 g, s = 700.5 g. Use a confidence level of 99% to complete parts (a) through (d) below. a. Identify the...
-
Simplify. (1 + i/i) -n
-
1) The government decided to reduce taxes on fast-food to increase revenue. The government assumes that fast-food products have a) An inelastic demand b) An elastic demand c) A demand curve that is...
-
Octane gas (C8H18) at 25oC is burned steadily with 30 percent excess air at 25oC, 1 atm, and 60 percent relative humidity. Assuming combustion is complete and adiabatic, calculate the exit...
-
Reconsider Prob. 15-75. Using EES (or other) software, investigate the effect of the relative humidity on the exit temperature of the product gases. Plot the exit temperature of the product gases as...
-
A coal from Pennsylvania has an ultimate analysis (by mass) as 84.36 percent C, 1.89 percent H2, 4.40 percent O2, 0.63 percent N2, 0.89 percent S, and 7.83 percent ash (non-combustibles) is burned in...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


