Determine the temperature at which 10 percent of diatomic hydrogen (H 2 ) dissociates into monatomic hydrogen
Question:
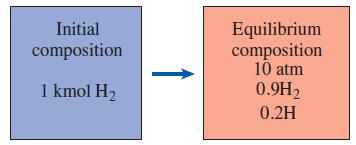
Determine the temperature at which 10 percent of diatomic hydrogen (H2) dissociates into monatomic hydrogen (H) at a pressure of 10 atm.
Transcribed Image Text:
Initial Equilibrium composition 10 atm 0.9H2 composition 1 kmol H2 0.2H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
The temperature at which 10 percent of H 2 dissociates into 2H is to be determined Assumptions 1 The ...View the full answer

Answered By

Kalyan M. Ranwa
I have more than seven years of teaching experience in physics and mechanical engineering.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
Determine the temperature at which 5 percent of diatomic oxygen (O2) dissociates into monatomic oxygen (O) at a pressure of 3 atm.
-
Determine the temperature at which 5 percent of diatomic oxygen (O2) dissociates into monatomic oxygen (O) at a pressure of 3 atm.
-
A mixture of 1 mol of H2 and 1 mol of Ar is heated at a constant pressure of 1 atm until 15 percent of H2 dissociates into monatomic hydrogen (H). Determine the final temperature of the mixture.
-
101, 115, 143, 106, 100, 142, 157, 163, 155, 141, 145, 153, 152, 147, 143, 115, 164, 160, 147, 150 (90%) Find the confidence interval of the median, indicated in parentheses, for the set of data.
-
The amount of milk obtained from a cow
-
Application: ToCallorNotToCall...
-
1 A continuacin se enlistan tres productos en diversas etapas de su ciclo de vida. Qu estrategias de marketing le recomendara a estas empresas?: a) cmaras digitales Canon, etapa de crecimiento ; b)...
-
Dana Baird was manager of a new Medical Supplies Division. She had just finished her second year and had been visiting with the companys vice president of operations. In the first year, the operating...
-
Compute the interest on a 15-year loan for $15023 if the annual rate is 4.9% with continuous compounding.
-
PQR Limited is an engineering company engaged in the manufacture of components and finished products. The company is highly mechanized and each of the components and finished products requires the...
-
The equilibrium constant for C + 1/2 O 2 CO 2 reaction at 100 kPa and 1600 K is K p . Use this information to find the equilibrium constant for the following reactions at 1600 K. (a) C + 1/2 O 2 CO...
-
At what temperature will oxygen be 15 percent disassociated at (a) 3 psia (b) 100 psia?
-
For the reaction 2 NO(g) + Cl 2 (g) 2 NOCl(g), K c = 3.7 x 10 8 at 298 K. In a 1.50 L flask, there are 4.125 mol of NOCl and 0.1125 mol of Cl 2 present at equilibrium (298 K). (a) Determine the...
-
Assume that John wants to annuitize the annuity and is told that he can receive a straight life annuity for $600 a month for life. If the actuarial number of payments is 300, how much of the first...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 90% confident that the estimated percentage is in error...
-
Your homework for this week is to watch the first lecture on Financial Accounting and at the end of the outline there are several problems for you to do. The problems begin with parts A-D for you to...
-
Sheril Rose was a brilliant but penniless material scientist. She had designed a new type of solar panel she believed had great commercial potential. On January 15, she approached Felda Higgins, a...
-
IAS 23 requires companies to capitalize borrowing costs directly attributable to the acquisition, construction or production of an asset into the cost of an asset.Previously, accounting standard...
-
Substitution and evaluation. Evaluate each of the following for the values given. I/Pt for I = 63, P = 840, t = 219/365
-
Flicker, Inc., a closely held corporation, acquired a passive activity this year. Gross income from operations of the activity was $160,000. Operating expenses, not including depreciation, were...
-
A constant-volume tank contains a mixture of 120 g of methane (CH4) gas and 600 g of O2 at 25oC and 200 kPa. The contents of the tank are now ignited, and the methane gas burns completely. If the...
-
Reconsider Prob. 15-65. Using EES (or other) software, investigate the effect of the final temperature on the final pressure and the heat transfer for the combustion process. Let the final...
-
One lbmol of methane (CH4) undergoes complete combustion with stoichiometric amount of air in a rigid container. Initially, the air and methane are at 14.4 psia and 77oF. The products of combustion...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


