Repeat the calculation in Problem 10.3 with the assumption that the heat capacity of liquid toluene is
Question:
Repeat the calculation in Problem 10.3 with the assumption that the heat capacity of liquid toluene is constant, using the values at
(a) 10◦C;
(b) 35◦C;
(c) 60◦C. How large is the error in each case? Give a quantitative explanation to the answer to part (b).
Problem 10.3
The data in Table 10.P2 are from a batch experiment measuring the distribution of octanoic acid between an aqueous phase consisting of a solution of corn syrup in water and an organic xylene phase. The aqueous phase was continuous, and the concentration in the aqueous phase was measured with a calibrated conductivity probe. Initially, 2 L of the aqueous phase was placed in a tank with 0.2 L of xylene and agitated until the drop size of the dispersed organic phase had equilibrated. 0.25 L of an aqueous phase containing octanoic acid was then added and the concentration in the continuous phase was recorded as a function of time. Determine Kma and compare to the value of 75 cm3/s reported by the authors of the original article.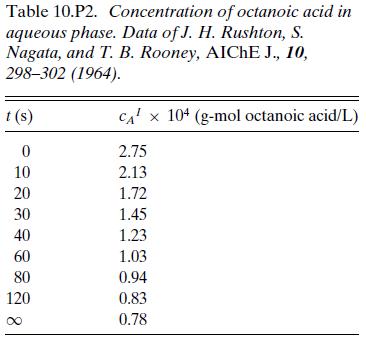
Step by Step Answer:






