Give IUPAC names for the following cycloalkanes: (c) CH2CH2CH3 (b) CH (a) CH CH3 (f) Br (e)
Question:
Give IUPAC names for the following cycloalkanes:
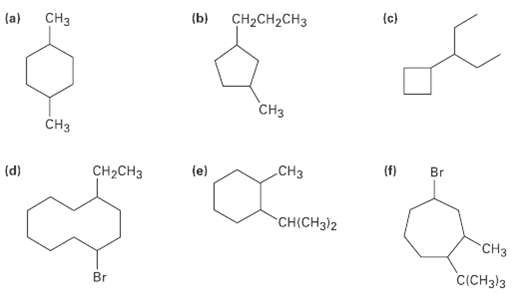
Transcribed Image Text:
(c) CH2CH2CH3 (b) CHз (a) CHз CH3 (f) Br (e) Cнз CH-CHз (d) CHICH3)2 "CHз C(CH3)3 Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Strategy The steps for naming a ...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give IUPAC names for the following alkenes: (a) (b) (c) (d) (e) (f) OH CI
-
Give IUPAC names for the following compounds. (a) (b) (c) (d) (e) (f) Ph CH3C C CH CH H3C CH3 - (CH3)3C C-CH (CH3)CH2CH3 CH CHC CC-OH CH,CH CH C-C CH
-
Give IUPAC names for the following compounds. (a) (b) (c) CH3 CH CH CH CH, CH, CH CH
-
Use a software package such as Matlab or Mathematica to program the example described in section 7.3. (a) Assume the environmental regulator ignores the impact on the labor market and sets an...
-
Ted Paulson needed money to pay for unexpected medical bills. To obtain $6,000, he decided to sale some of his shares in the Ridgemoor Capital Appreciation Fund. When he called the investment...
-
In the buying center, the gatekeeper controls information flow to others in the center. Thus, the gatekeeper determines which possible sellers are heard and which are not. Does the gatekeeper have...
-
Sally Cook, Lin Xi, and Ken Schwartz formed the CXS Partnership by making capital contributions of $144,000, $216,000, and $120,000, respectively. They predict annual partnership net income of...
-
Nuncio Consulting completed the following transactions during June. a. Armand Nuncio, the owner, invested $35,000 cash along with office equipment valued at $11,000 in the new company. b. The company...
-
On January 1, 2019, YOU T00 Corporation purchased P1,000,000 10% bonds classified as FA@AC. The bonds were purchased to yield 12%. Interest is payable annually every December 31. The bonds mature on...
-
Profits have been decreasing for several years at Pegasus Airlines. In an effort to improve the company?s performance, consideration is being given to dropping several flights that appear to be...
-
We?ll see that there are two isomeric substances both named 1, 2-dimethylcyclohexane. Explain. -C3 1,2-Dimethylcyclohexane CH
-
Draw structures corresponding to the following IUPAC names: (a) 1, 1-Dimethylcycloocatne (b) 2-Cyclobutylhexane (c) 1, 2-Dichlorocyclopentane (d) 1, 3-Dibromo-5-methylcyclohexane
-
According to the law of large numbers, as the number of insureds increases, risk is reduced. However, as an insurance company writes more policies, it exposes itself to the potential for greater...
-
reflective account of your development as a postgraduate learner since joining SBS considering the points below. Critically reflect on one or more points below: Assessment Criteria Use a reflective...
-
Technology, strategy, size, and environment are among the factors that influence leaders' choice of organization structure (Schulman, 2020). The leaders must consider the technology to be used in the...
-
6. Answer the following briefly. a.What is the metric and its hurdle rate for an "Enterprise" to increase its enterprise value? b.What is the metric and its hurdle rate for the corporation's equity...
-
Name the two major preceding management theories that contributed to the development of quality management theory. Briefly explain the major concepts of each of these preceding theories that were...
-
922-19x 8 After finding the partial fraction decomposition. (22 + 4)(x-4) dx = dz Notice you are NOT antidifferentiating...just give the decomposition. x+6 Integrate -dx. x33x The partial fraction...
-
write a final project report that presents an authoritative account of your research. LO3
-
Respond to the ethical judgments required based on the following scenarios. Scenario 1. Assume you have collected a sample using MUS and that you have evaluated that sample to calculate a total...
-
The vapor pressure of methyl alcohol is 40.0 mmHg at 5.0 C. Use this value and other information from the text to estimate the normal boiling point of methyl alcohol.
-
The following reaction is the first step in the industrial synthesis of acetone and phenol (C6H5OH). AIBN (2,29-azobisisobutyronitrile) initiates radical reactions by breaking down upon heating to...
-
In the radical chlorination of 2,2-dimethylhexane, chlorine substitution occurs much more rapidly at C5 than it does at a typical secondary carbon (e.g., C2 in butane). Consider the mechanism of...
-
Write a mechanism for the following reaction. (PhCO2)2, heat + CO
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


