Question: The following specific reaction rate constant data were reported by Smith [J. Phys. & Coll. Chem., 59, 367 (1947)] for the reaction urea + formaldehyde
The following specific reaction rate constant data were reported by Smith [J. Phys. & Coll. Chem., 59, 367 (1947)] for the reaction urea + formaldehyde → monomethylolurea: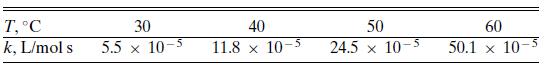
Check the validity of the Arrhenius relation and determine the activation energy.
T, C k, L/mol s 30 5.5 x 10-5 40 11.8 x 10-5 50 24.5 x 10-5 60 50.1 x 10-5
Step by Step Solution
3.32 Rating (155 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


