Water originally saturated with carbon tetrachloride (left(mathrm{CCl}_{4} ight)) at (25.0^{circ} mathrm{C}) and (1.0 mathrm{~atm}) is stripped with
Question:
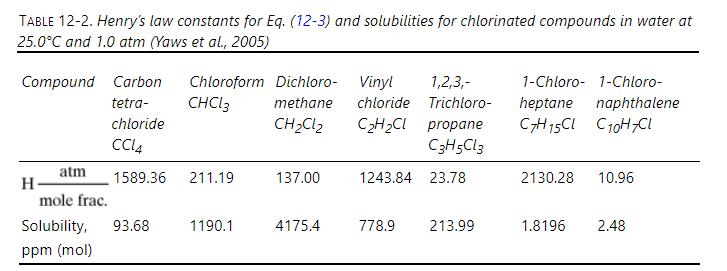
Water originally saturated with carbon tetrachloride \(\left(\mathrm{CCl}_{4}\right)\) at \(25.0^{\circ} \mathrm{C}\) and \(1.0 \mathrm{~atm}\) is stripped with pure air at \(25.0^{\circ} \mathrm{C}\) and \(745 \mathrm{~mm} \mathrm{Hg}\). Exit water has \(1.0 \mathrm{ppm}\) (mole) of \(\mathrm{CCl}_{4}\). Operation is at \(\mathrm{L} / \mathrm{V}=0.8(\mathrm{~L} / \mathrm{V})_{\max }\). If \(\mathrm{H}_{\mathrm{OL}}=0.62 \mathrm{~m}\), find the height of packing. Data are in Table 12-2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





