We are separating ethanol and water. All percentages are mol%. Column pressure is at (1.0 mathrm{~atm}). VLE
Question:
We are separating ethanol and water. All percentages are mol\%. Column pressure is at \(1.0 \mathrm{~atm}\). VLE data are in Table 2-1. Find the q values and plot the feed lines for the following situations:
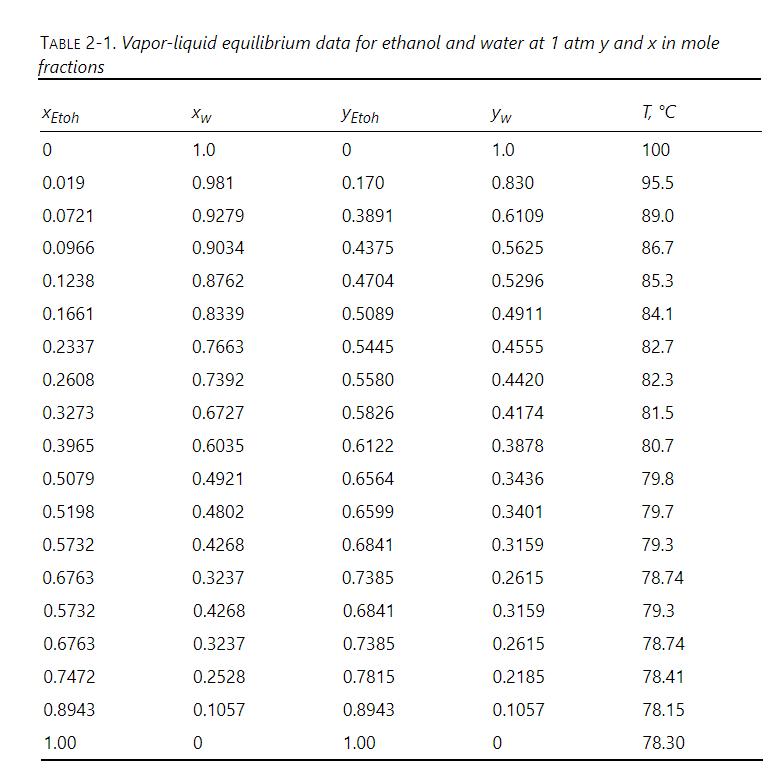
a. Feed is \(60 \%\) ethanol and flashes in the column with \(\mathrm{V} / \mathrm{F}=0.37\).
b. Feed is \(40 \%\) ethanol and is a two-phase mixture with liquid and vapor in equilibrium at a temperature of \(84.1{ }^{\circ} \mathrm{C}\).
c. Feed is \(40 \%\) ethanol and is a liquid at \(20^{\circ} \mathrm{C}\).
d. Feed is \(40 \%\) ethanol and is a vapor at \(120^{\circ} \mathrm{C}\).
e. Feed is \(50 \%\) ethanol and is a subcooled liquid. One mole of vapor must be condensed to heat 12 moles of feed to their boiling point.
f. Feed is \(20 \%\) ethanol, and \(70 \%\) is vaporized in a flash distillation system. The products of the flash system are fed separately to different feed stages. Calculate the two \(\mathrm{q}\) values, and plot the two feed lines.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat




