We have (100 mathrm{~kg} / mathrm{h}) of a feed that is (60 mathrm{wt} %) methylcyclohexane (A) and
Question:
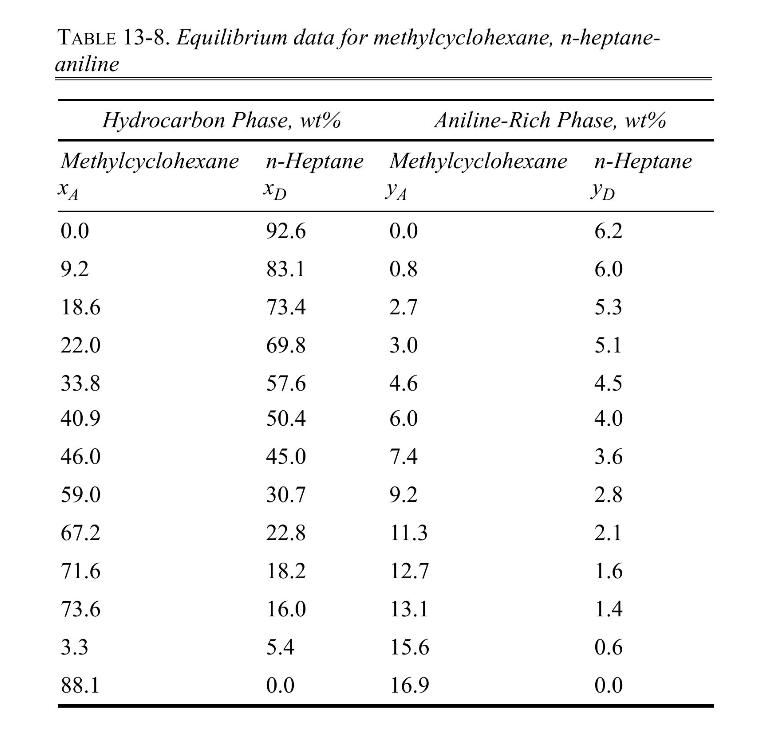
We have \(100 \mathrm{~kg} / \mathrm{h}\) of a feed that is \(60 \mathrm{wt} \%\) methylcyclohexane (A) and \(40 \mathrm{wt} \% \mathrm{n}\)-heptane (D) and \(50 \mathrm{~kg} / \mathrm{h}\) of a feed that is \(20 \mathrm{wt} \%\) methylcyclohexane and \(80 \mathrm{wt} \% \mathrm{n}\)-heptane. These two feeds are mixed with \(200 \mathrm{~kg} / \mathrm{h}\) of pure aniline (S) in a single equilibrium stage. Equilibrium data for extraction of methylcyclohexane (A) from nheptane (D) into aniline (S) are given in Table 13-8.
a. What are extract and raffinate compositions leaving the stage?
b. What is the flow rate of extract product?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





