Question: We have a feed that is a binary mixture of methanol and water (55.0 mol% methanol) that is sent to a system of two flash
We have a feed that is a binary mixture of methanol and water (55.0 mol% methanol) that is sent to a system of two flash drums hooked together. The vapor from the first drum is cooled, which partially condenses the vapor, and then is fed to the second flash drum. Both drums operate at a pressure of 1.0 atm and are adiabatic. The feed rate to the first drum is 1000.0 kmol/h. We desire a liquid product from the first drum that is 30.0 mol% methanol (x1 = 0.300). The second drum operates at a fraction vaporized of (V/F)2 = 0.250. The equilibrium data are in Table 2-8 in Problem 2.D1.
Table 2-8
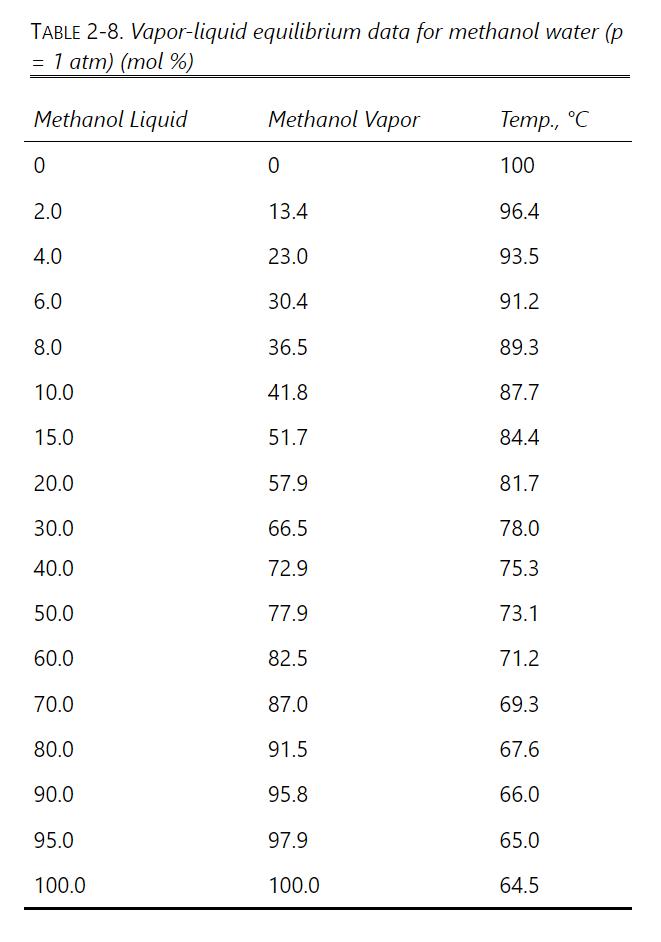
a. Find the following for the first drum: y1,T1 ,(V/F)1, and vapor flow rate V1.
b. Find the following for the second drum: y2, X2, T2, and vapor flow rate V2.
Problem 2.D1
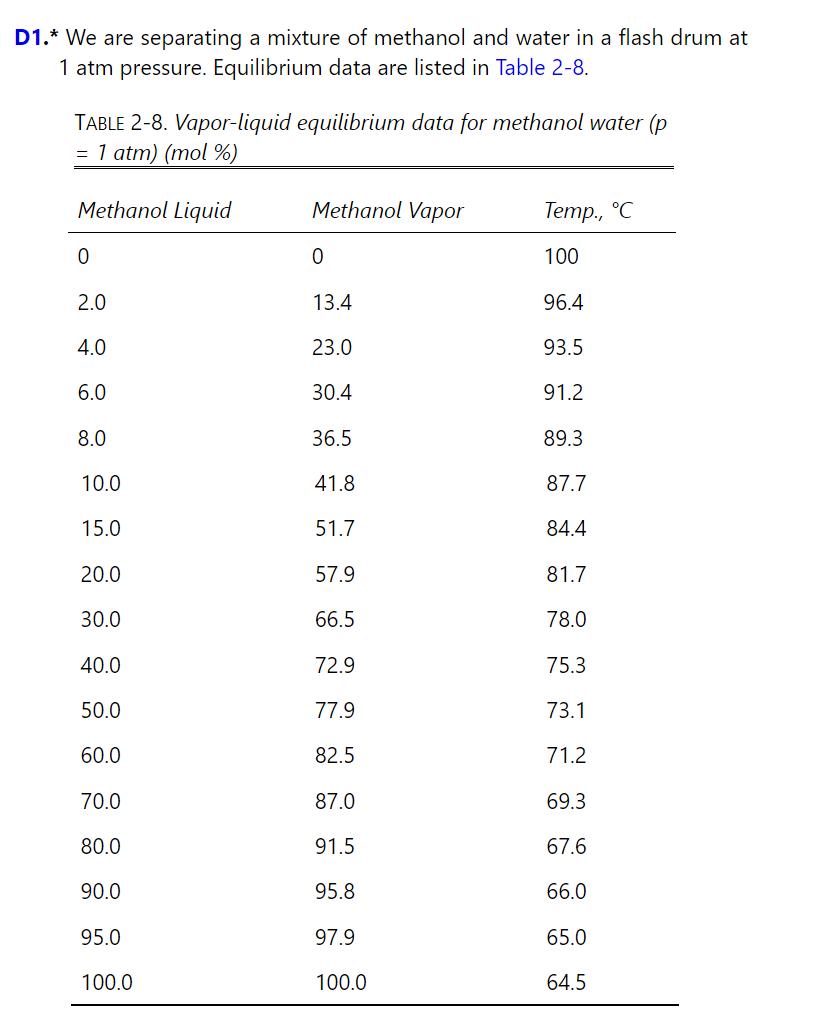
0 TABLE 2-8. Vapor-liquid equilibrium data for methanol water (p = 1 atm) (mol %) Methanol Liquid 0 Methanol Vapor Temp., C 100 2.0 13.4 96.4 4.0 23.0 93.5 6.0 30.4 91.2 8.0 36.5 89.3 10.0 41.8 87.7 15.0 51.7 84.4 20.0 57.9 81.7 30.0 66.5 78.0 40.0 72.9 75.3 50.0 77.9 73.1 60.0 82.5 71.2 70.0 87.0 69.3 80.0 91.5 67.6 90.0 95.8 66.0 95.0 97.9 65.0 100.0 100.0 64.5
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


