We mix (1100 mathrm{~kg} / mathrm{h}) of water at (100^{circ} mathrm{C}) with (750 mathrm{~kg} / mathrm{h}) of
Question:
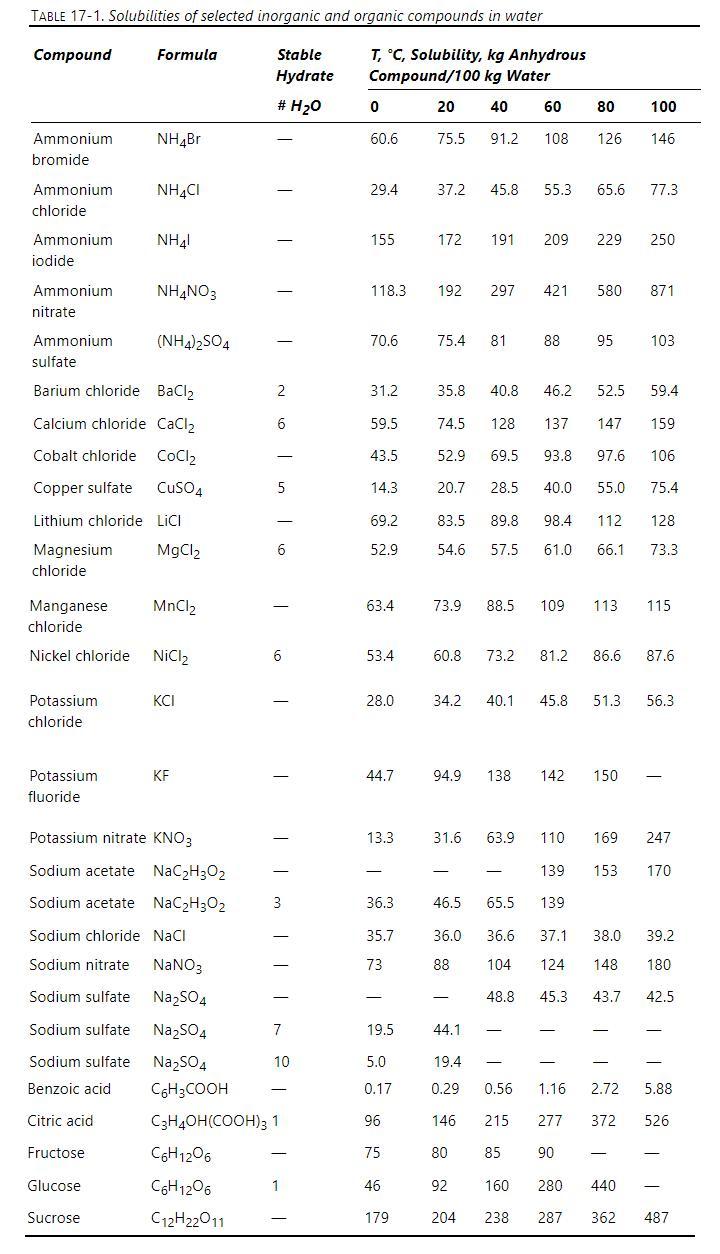
We mix \(1100 \mathrm{~kg} / \mathrm{h}\) of water at \(100^{\circ} \mathrm{C}\) with \(750 \mathrm{~kg} / \mathrm{h}\) of copper sulphate hydrate crystals. This mixture is cooled to \(10^{\circ} \mathrm{C}\) while evaporating \(120 \mathrm{~kg} / \mathrm{h}\) of water under a vacuum.
a. How many \(\mathrm{kg} / \mathrm{h}\) of hydrated crystals are collected?
b. What mass, \(\mathrm{kg} / \mathrm{h}\), of anhydrous crystals are collected?
Data: Solubility data are in Table 17-1. \(\mathrm{MW}: \mathrm{Cu}=63.5, \mathrm{~S}=32.1, \mathrm{O}=16, \mathrm{H}=1\).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat
Question Posted:





