We wish to do a constant mole batch distillation of 100 moles of feed that is pure
Question:
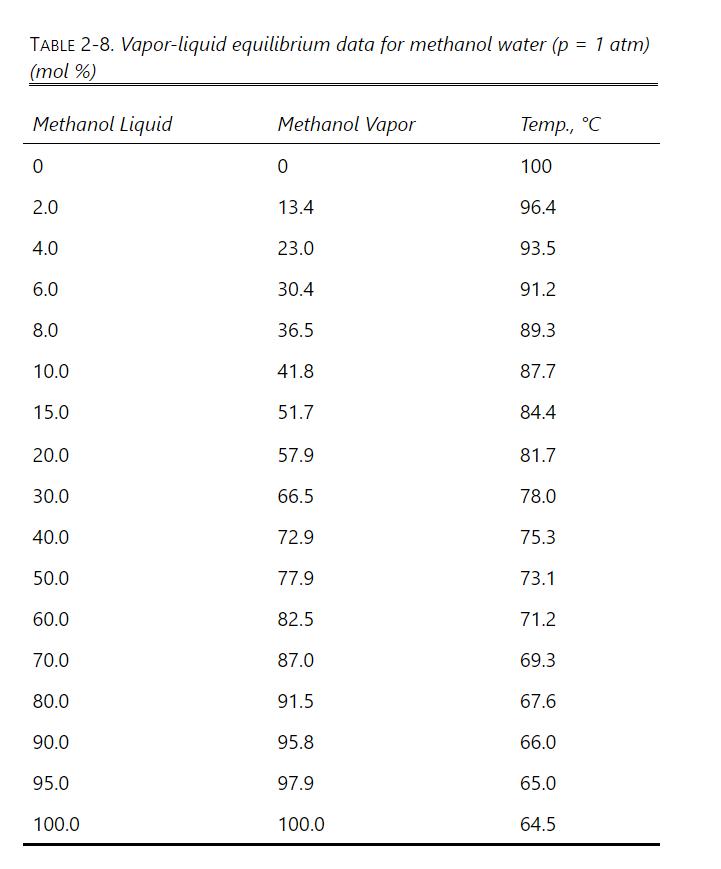
We wish to do a constant mole batch distillation of 100 moles of feed that is pure water \(\left(x_{W}=1\right)\) to exchange the solvent to obtain the nonvolatile solute in a mixture that is 0.01 mole fraction water and 0.99 mole fraction methanol. Develop a spreadsheet for binary batch, constant mole distillation that uses Eq. (9-18). Because \(\alpha\) is not constant, calculate the values of S/W over a series of relatively small ranges of \(x\) using the geometric average \(\alpha\) value for each range (e.g., for range \(\mathrm{x}_{\mathrm{W}}=0.9\) to 0.7,\(\left.\alpha_{\text {avg }}=\left[\alpha_{\mathrm{w}}(0.9) \alpha_{\mathrm{w}}(0.8) \alpha_{\mathrm{w}}(0.7)\right]^{0.3333}\right)\). Use the equilibrium data in Table \(2-8\) to calculate \(\alpha\) values. Find \(S, D_{\text {total }}\), and \(x_{D, a v g}\).
1 . Because this is a constant mole process, \(\mathrm{W}\) is constant \(=\mathrm{F}\).
2. \(\alpha_{\text {Water } \mathrm{M}}>1.0\).
Equation 9-18

Table 2-8
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





