Question:
What is the Fickian diffusivity of chlorobenzene in liquid bromobenzene at \(300 \mathrm{~K}\) when the mole fraction of chlorobenzene is 0.0332 ? Assume that the diffusivity follows an Arrhenius form and use the data in Table 15-3 to determine \(\mathrm{E}_{\mathrm{o}}\). Also report the value of \(\mathrm{E}_{\mathrm{o}}\) in \(\mathrm{J} / \mathrm{mol}\).
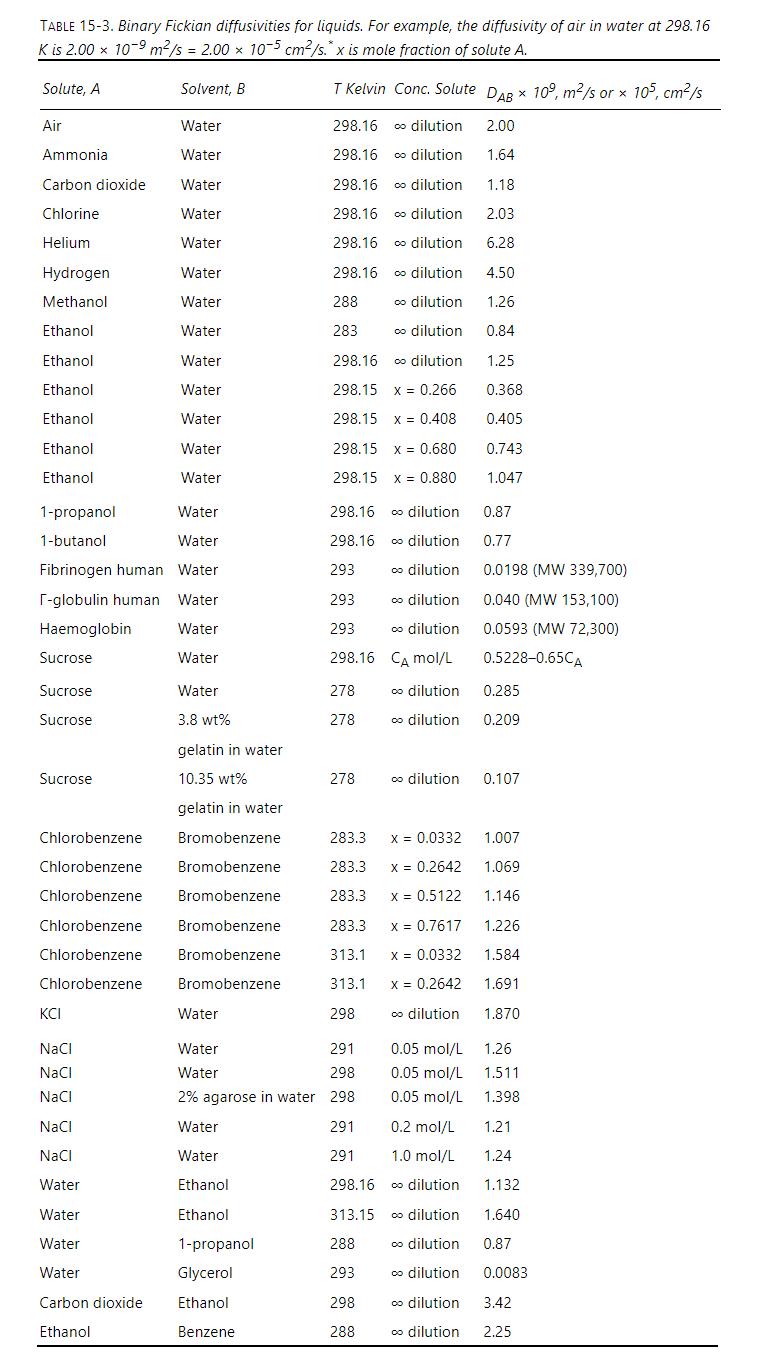
Transcribed Image Text:
TABLE 15-3. Binary Fickian diffusivities for liquids. For example, the diffusivity of air in water at 298.16 K is 2.00 10-9 m/s = 2.00 x 10-5 cm/s.* x is mole fraction of solute A. Solute, A Solvent, B T Kelvin Conc. Solute DAB 109, m2/s or 105, cm/s Air Water 298.16 co dilution 2.00 Ammonia Water 298.16 co dilution 1.64 Carbon dioxide Water 298.16 co dilution 1.18 Chlorine Water 298.16 co dilution 2.03 Helium Water 298.16 co dilution 6.28 Hydrogen Water 298.16 co dilution 4.50 Methanol Water 288 co dilution 1.26 Ethanol Water 283 co dilution 0.84 Ethanol Water 298.16 co dilution 1.25 Ethanol Water 298.15 x 0.266 0.368 Ethanol Water 298.15 x 0.408 0.405 Ethanol Water 298.15 x 0.680 0.743 Ethanol Water 298.15 x 0.880 1.047 1-propanol Water 298.16 co dilution 0.87 1-butanol Water 298.16 co dilution 0.77 Fibrinogen human Water 293 dilution 0.0198 (MW 339,700) T-globulin human Water 293 dilution 0.040 (MW 153,100) Haemoglobin Water 293 co dilution 0.0593 (MW 72,300) Sucrose Water 298.16 CA mol/L 0.5228-0.65CA Sucrose Water 278 dilution 0.285 Sucrose 3.8 wt% 278 dilution 0.209 gelatin in water Sucrose 10.35 wt% 278 dilution 0.107 gelatin in water Chlorobenzene Bromobenzene 283.3 x = 0.0332 1.007 Chlorobenzene Bromobenzene 283.3 x = 0.2642 1.069 Chlorobenzene Bromobenzene 283.3 x = 0.5122 1.146 Chlorobenzene Bromobenzene 283.3 x = 0.7617 1.226 Chlorobenzene Bromobenzene 313.1 x = 0.0332 1.584 Chlorobenzene Bromobenzene 313.1 x = 0.2642 1.691 KCI Water 298 dilution 1.870 NaCl Water 291 0.05 mol/L 1.26 NaCl Water 298 0.05 mol/L 1.511 NaCl 2% agarose in water 298 0.05 mol/L 1.398 NaCl Water 291 0.2 mol/L 1.21 NaCl Water 291 1.0 mol/L 1.24 Water Ethanol 298.16 co dilution 1.132 Water Ethanol 313.15 co dilution 1.640 Water 1-propanol 288 dilution 0.87 Water Glycerol 293 co dilution 0.0083 Carbon dioxide Ethanol 298 dilution 3.42 Ethanol Benzene 288 dilution 2.25








