Your boss wants some idea of how expensive it will be to distill 155.0 (mathrm{kmol} / mathrm{h})
Question:
Your boss wants some idea of how expensive it will be to distill 155.0 \(\mathrm{kmol} / \mathrm{h}\) of a saturated liquid feed that is \(5.0 \mathrm{~mol} \%\) methane, \(10.0 \mathrm{~mol} \%\) ethane, \(15.0 \mathrm{~mol} \%\) n-butane, \(22.0 \mathrm{~mol} \% \mathrm{n}\)-pentane, \(22.0 \mathrm{~mol} \% \mathrm{n}-\) hexane, and \(26.0 \mathrm{~mol} \% \mathrm{n}\)-heptane. Column pressure is \(700.0 \mathrm{kPa}\). The column has a partial condenser and a partial reboiler. We want to recover \(99.0 \%\) of the \(n\)-butane in the distillate and \(98.3 \%\) of the \(n-\) pentane in the bottoms. Do the calculations of the \(\mathrm{K}\) values either from the DePriester chart or from Eq. (2-28).
Equation (2-28)
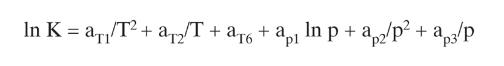
a. Assuming that NKs do not distribute, calculate the values of \(\mathrm{D}\) and \(\mathrm{B}\) in \(\mathrm{kmol} / \mathrm{h}\) and the mole fractions in distillate and bottoms.
b. Do a bubble-point calculation at the feed conditions. Calculate the relative volatilities of all components with respect to the HK (npentane). Use these values as the average value of relative volatility for the entire column. Also determine the bubble-point temperature of the distillate to see if condensation will be expensive.
c. Determine the minimum number of stages for this separation with the Fenske equation.
d. Determine the minimum reflux ratio, \((\mathrm{L} / \mathrm{D})_{\min }\), with the Underwood method.
e. Estimate the number of stages required if \(\mathrm{L} / \mathrm{D}=\mathrm{M} \times(\mathrm{L} / \mathrm{D})_{\min }\) with the Gilliland correlation (Davis's fit is convenient) where M \(=1.04,1.10\), and 2.0 .
f. Will this distillation be reasonably economical, or should an alternative be found? Briefly explain your reasoning.
Parts b and \(\mathrm{d}\) are easier to do with a spreadsheet or Wolfram.
Step by Step Answer:

Separation Process Engineering Includes Mass Transfer Analysis
ISBN: 9780137468041
5th Edition
Authors: Phillip Wankat





