Question: A multicomponent mixture is boiled in a flask at (1 mathrm{~atm}). The vapors are condensed and recovered as a liquid product. It is desired to
A multicomponent mixture is boiled in a flask at \(1 \mathrm{~atm}\). The vapors are condensed and recovered as a liquid product. It is desired to examine the mole fractions of the residual liquid in the flask as vaporization proceeds. Although sketches of the residue-curve maps are called for in (b)-(d), a process simulator can be used to prepare the drawings accurately.
(a) For a mixture of \(60 \mathrm{~mol} \% \mathrm{n}\)-butane (1) and \(40 \mathrm{~mol} \% \mathrm{n}\)-pentane (2), determine the residual mole fraction of \(n\)-butane after \(10 \%\) of the liquid has vaporized.
(b) Consider mixtures of n-butane (1), n-pentane (2), and n-hexane (3). For three typical feed compositions:
Sketch the residue curves (solutions of the ODEs; do not solve them analytically or numerically) on triangular graph paper. Use arrows to show the direction along the trajectories in time.
(c) Repeat
(b) for mixtures of acetone (1), choroform (2), and benzene (3). Note that the acetone-chloroform binary exhibits a maximum-boiling azeotrope \(\left(64^{\circ} \mathrm{C}\right)\) at \(35 \mathrm{~mol} \%\) acetone with no other azeotropes existing. Sketch any boundaries across which the residue curves cannot traverse.
(d) Repeat
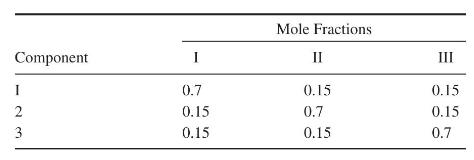
(c) for mixtures of methyl acetate (1), methanol (2), and \(\mathrm{n}\)-hexane (3). Note the existence of four azeotropes, where compositions are in \(\mathrm{mol} \%\).
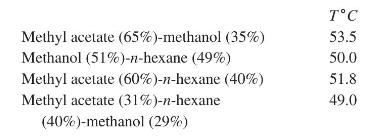
Mole Fractions Component I II III I 0.7 0.15 0.15 23 0.15 0.7 0.15 0.15 0.15 0.7
Step by Step Solution
3.42 Rating (155 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


