Question: Alter the design of the cyclohexane process in Example 10.5 to reduce the lost work and increase the thermodynamic efficiency. Use a simulation program to
Alter the design of the cyclohexane process in Example 10.5 to reduce the lost work and increase the thermodynamic efficiency. Use a simulation program to complete the material and energy balances, and compute the entropies and availability functions for all of the streams as well as the lost work for each piece of equipment.
Data From Example 10.5:-

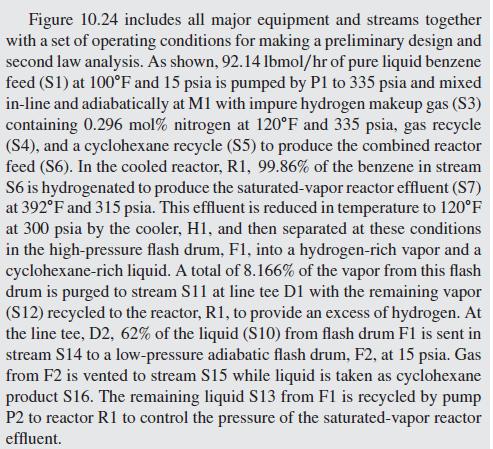

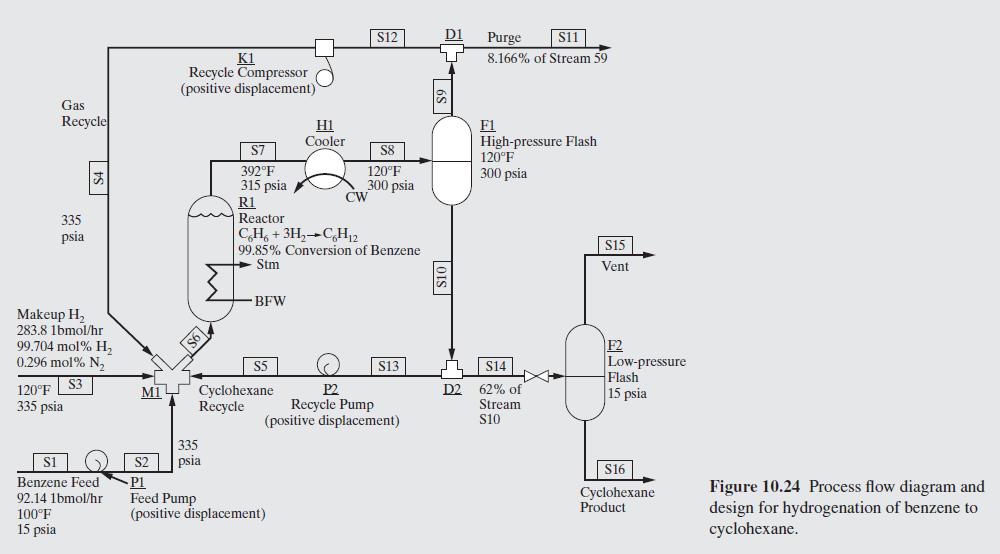
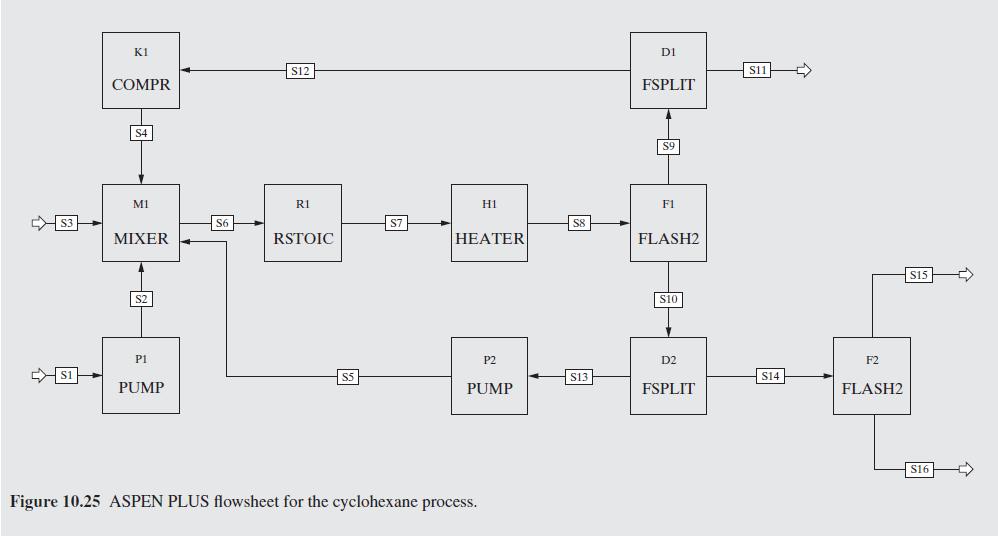
Figure 10.26:-
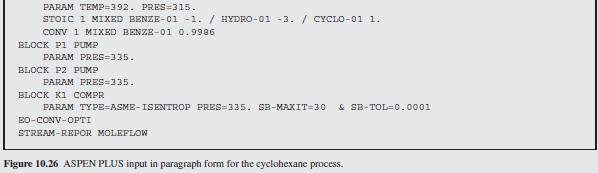
Figure 10.27:-
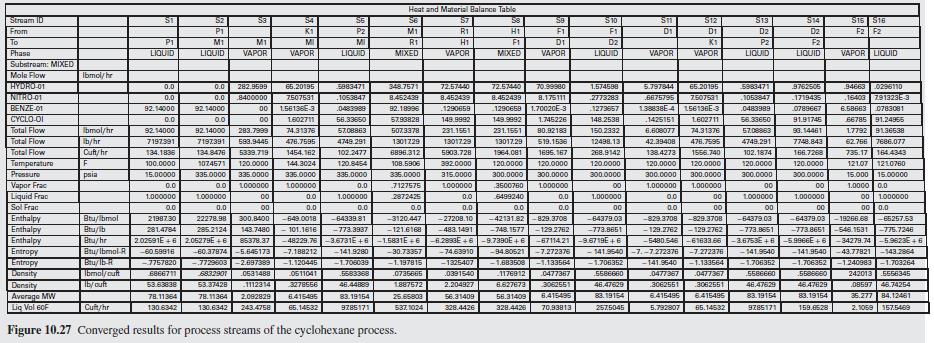
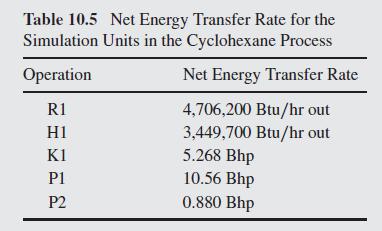
Table 10.5:-
Equation 10.24:-

Here, a process is considered that involves a chemical reactor as well as separators, heat exchangers, and pumps. A continuous, steady-state, steady-flow process for manufacturing approximately 10 million gal- lons per year of high-purity cyclohexane by the catalytic hydrogena- tion of high-purity benzene, at elevated temperature and pressure, is shown in Figure 10.24. The heart of the process is a reactor in which liquid benzene from storage, together with makeup hydrogen and recy- cle hydrogen in stoichiometric excess, take part in the reaction: C6H6 + 3H2C6H12
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


