Calculate the standard heat of reaction for the following reaction: the hydrogenation of benzene to cyclohexane. (1)
Question:
Calculate the standard heat of reaction for the following reaction: the hydrogenation of benzene to cyclohexane.
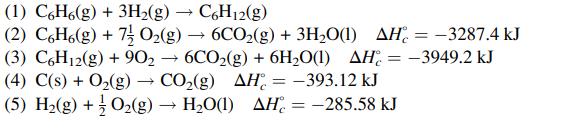
Transcribed Image Text:
(1) C6H6(g) + 3H₂(g) → C6H12(g) (2) C6H6(g) + 7 O₂(g) 6CO₂(g) + 3H₂O(1) AH-3287.4 kJ (3) C6H12(g) + 90₂ → 6CO₂(g) + 6H₂O(1) AH-3949.2 kJ CO₂(g) AH = -393.12 kJ (4) C(s) + O₂(g) (5) H₂(g) + O₂(g) H₂O(1) AH= -285.58 kJ → -
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Method 1 Using the more general equation 326 ...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler
Question Posted:
Students also viewed these Engineering questions
-
All chemists know that benzene is unusually stable, that is, it is aromatic. They are also well aware that many other similar molecules are stabilized by aromaticity to some extent and, more often...
-
Calcium chloride is a salt used in a number of food and medicinal applications and in brine for refrigeration systems. Its most distinctive property is its affinity for water: in its anhydrous form...
-
The standard heat of combustion of gaseous acetylene is listed in Table B.I as 1299.6kJ/mol. (a) In your own words, briefly explain what that means. (Your explanation should mention the reference...
-
Rainfall of magnitude 3.8cm and 2.8cm occurring on two consecutive 4-h durations on a catchment of area 27km produced the following hydrograph of flow at the outlet of the catchment. Estimate the...
-
The following summary data are from the May 31, 2004, balance sheet of FedEx. All numbers are in millions. Total current assets. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $ 4,970...
-
Distinguish between hedgers, speculators, and arbitrageurs.
-
Diversity training is becoming more important in the workplace. However, many people dislike or resist diversity training. Why do you think some resist this training? What can companies and managers...
-
Russia Spring produces premium bottled water. Russia Spring purchases artesian water, stores the water in large tanks, and then runs the water through two processes: filtration and bottling. During...
-
Jakes Roof Repair has provided the following data concerning its costs: Fixed Cost per Month Cost per Repair-Hour Wages and salaries $ 21,400 $ 15.00 Parts and supplies $ 7.70 Equipment depreciation...
-
AT&T plans to sell the new iPhone for $200. The lower-priced phone would give AT&T an attractive weapon to win new subscribers. AT&Ts revenue is an average of $95 a month from each iPhone customer,...
-
Illustrates the manual calculation of a reactor heat balance. Vinyl chloride (VC) is manufactured by the pyrolysis of 1,2-dichloroethane (DCE). The reaction is endothermic. The flow-rates to produce...
-
Methane is compressed from 1 bar and 290 K to 10 bar. If the isentropic efficiency is 0.85, calculate the energy required to compress 10,000 kg/h. Estimate the exit gas temperature.
-
When the economy falters, consumers naturally tighten their belts. They drive fewer miles, make smaller purchases, and eat out less often. None of this is good news for the restaurant industry, which...
-
1. Using the net present value? method, calculate the comparative cost of each of the three payment plans being considered by New Med 2. Which payment plan should New Med choose? Explain. 3. Discuss...
-
Swenson Company produced 300 units in year one and sold 260 units in that year. In year two, it produced 260 units and sold 300 units. Total fixed overhead was the same in years one and two. Under...
-
c) Determine the maximum rotational speed such that the fluid will not spill over the container. (and: = 2gh/R) [2 marks] d) The container in Figure 4 now contains coffee (p~1000) which is 7cm deep...
-
FICO credit scores: x = 564,= 743,= 72 (Round your answer to 3 decimal places.) what does z equal
-
Q3: In the section illustrated in Figure (1) the surface 1-4-7 is insulated. The convection heat transfer coefficient at surface 1-2-3 is 28 W/m. 'C. The thermal conductivity of the solid material is...
-
A JFET (IDSS = 10mA, VP = -5V) is biased at ID = IDSS / 4. What is the value of gm at that bias point?
-
Thalina Mineral Works is one of the worlds leading producers of cultured pearls. The companys condensed statement of cash flows for the years 20182020 follows. Required Comment on Thalina Mineral...
-
Refer to the Fourier transform infrared spectrum in Figure 19-32. (a) The interferogram was sampled at retardation intervals of 1.2660 10 -4 cm. What is the theoretical wavenumber range (0 to ?) of...
-
The table shows signal-to-noise ratios recorded in a nuclear magnetic resonance experiment. Construct graphs of (a) signal-tonoise ratio versus n and (b) signal-to-noise ratio versus n, where n is...
-
Would you use a tungsten or a deuterium lamp as a source of 300-nm radiation? What kind of lamp provides radiation at 4-m wavelength?
-
Moore Corporation repurchased 3,700 shares of its own stock for $60 per share. The stock has a par of $15 per share. A month later Moore resold 925 shares of the treasury stock for $68 per share....
-
Vaughn Company applies overhead based on direct labour hours. Two direct labour hours are required for each unit of product. Planned production for the period was set at 8,500 units. Manufacturing...
-
Jane Doe takes out a $100,000 15-year loan from Acme Corporation at an annual effective rate of 6%. She can either repay her loan via the amortization method with level annual payments, or via the...

Study smarter with the SolutionInn App


