Illustrates the manual calculation of a reactor heat balance. Vinyl chloride (VC) is manufactured by the pyrolysis
Question:
Illustrates the manual calculation of a reactor heat balance.
Vinyl chloride (VC) is manufactured by the pyrolysis of 1,2-dichloroethane (DCE). The reaction is endothermic. The flow-rates to produce 5000 kg/h at 55 per cent conversion are shown in the diagram (see Example 2.13). The reactor is a pipe reactor heated with fuel gas, gross calorific value 33.5 MJ/m3 . Estimate the quantity of fuel gas required.
Data from example 2.13
The block diagram shows the main steps in the balanced process for the production of vinyl chloride from ethylene. Each block represents a reactor and several other processing units. The main reactions are:
Block A, chlorination
![]()
Block B, oxhydrochlorination
![]()
Block C, pyrolysis
![]()
The HCl from the pyrolysis step is recycled to the oxyhydrochlorination step. The flow of ethylene to the chlorination and oxyhydrochlorination reactors is adjusted so that the production of HCl is in balance with the requirement. The conversion in the pyrolysis reactor is limited to 55%, and the unreacted dichloroethane (DCE) is separated and recycled.
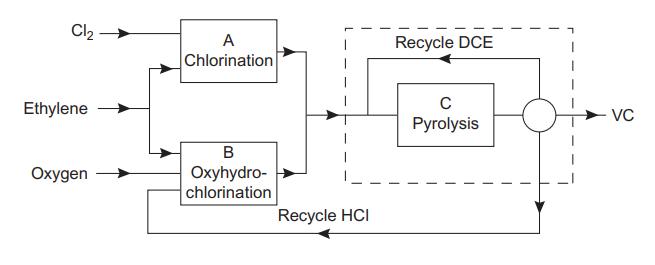
Using the yield figures given, and neglecting any other losses, calculate the flow of ethylene to each reactor and the flow of DCE to the pyrolysis reactor, for a production rate of 12,500 kg/h vinyl chloride (VC).

Step by Step Answer:

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler





