Question: Estimate the critical constants for diphenylmethane using Lydersens method; normal boiling point 537.5 K, molecular mass 168.2, structural formula: HC H H C. C H
Estimate the critical constants for diphenylmethane using Lydersen’s method; normal boiling point 537.5 K, molecular mass 168.2, structural formula:
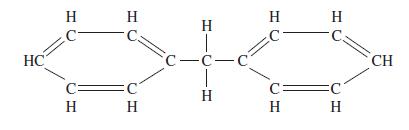
HC H H C. C H T c-- | H H C H CH
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
Group HCring Cring CHz No of 10 2 1 Total contr... View full answer

Get step-by-step solutions from verified subject matter experts


