A colleague has been doing an experiment in which it was important to know the mole fractions
Question:
A colleague has been doing an experiment in which it was important to know the mole fractions of the components of a solution. You want to use the same solution, but you have in mind an experiment in which it is necessary to know the molality of the solute. What is the molality of benzene, C6H6, dissolved in toluene, C6H5CH3, in a solution for which the mole fraction of benzene is 0.150?
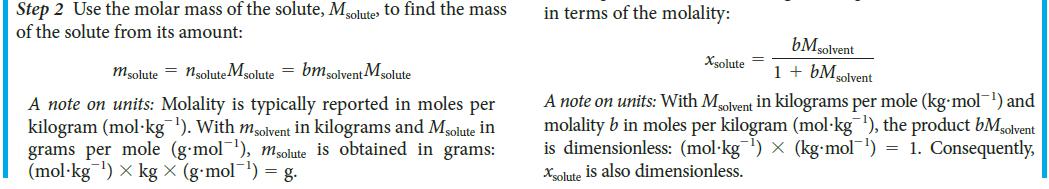
PLAN Use procedure 2 in Toolbox 5E.1, taking note of the discussion of units.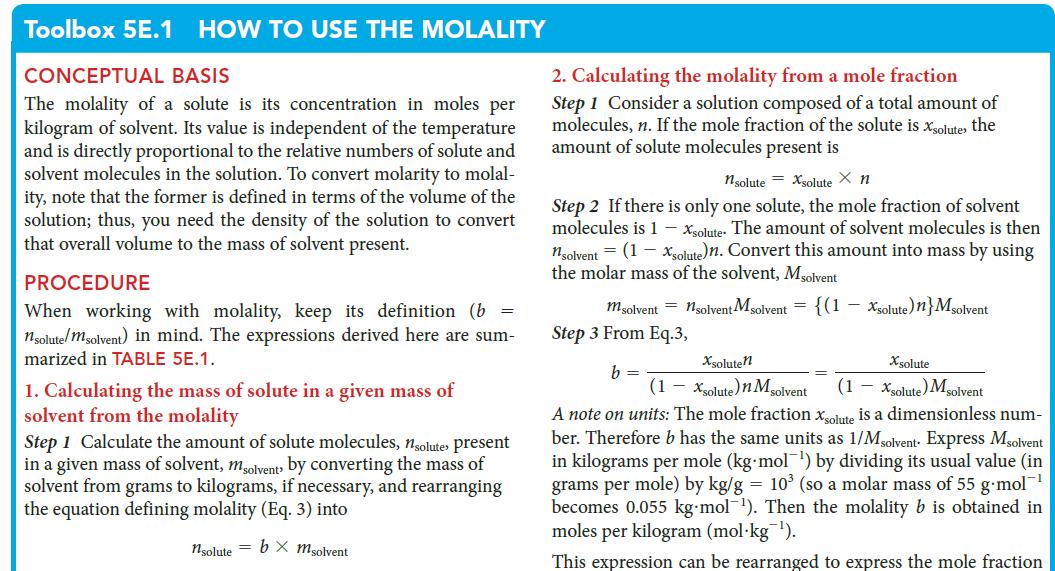
Transcribed Image Text:
Toolbox 5E.1 HOW TO USE THE MOLALITY CONCEPTUAL BASIS The molality of a solute is its concentration in moles per kilogram of solvent. Its value is independent of the temperature and is directly proportional to the relative numbers of solute and solvent molecules in the solution. To convert molarity to molal- ity, note that the former is defined in terms of the volume of the solution; thus, you need the density of the solution to convert that overall volume to the mass of solvent present. PROCEDURE When working with molality, keep its definition (b = nsolute/msolvent) in mind. The expressions derived here are sum- marized in TABLE 5E.1. 1. Calculating the mass of solute in a given mass of solvent from the molality Step 1 Calculate the amount of solute molecules, nsolutes present in a given mass of solvent, msolvent by converting the mass of solvent from grams to kilograms, if necessary, and rearranging the equation defining molality (Eq. 3) into nsolute = b x msolvent 2. Calculating the molality from a mole fraction Step 1 Consider a solution composed of a total amount of molecules, n. If the mole fraction of the solute is xsolute, the amount of solute molecules present is nsolute = xsolute X n Step 2 If there is only one solute, the mole fraction of solvent molecules is 1 - Xsolute. The amount of solvent molecules is then nsolvent = (1-xsolute)n. Convert this amount into mass by using the molar mass of the solvent, Msolvent m solvent = nsolvent Msolvent = {(1 - Xsolute) n} Msolvent Step 3 From Eq.3, Xsoluten Xsolute (1 - Xsolute) M solvent (1 - Xsolute) Msolvent A note on units: The mole fraction xsolute is a dimensionless num- ber. Therefore b has the same units as 1/Msolvent Express Msolvent in kilograms per mole (kg-mol¹) by div its usual value (in grams per mole) by kg/g = 10³ (so a molar mass of 55 g-mol™¹ becomes 0.055 kg-mol¹). Then the molality b is obtained in moles per kilogram (mol kg ¹). This expression can be rearranged to express the mole fraction b =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
A molar mass of 9213 gmol is equivalent to 0092 13 kgmol From b Xsolute1 Xsolute Msolvent with Msol...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In the Hollywood movie A Beautiful Mind, Russel Crowe plays John Nash who developed the Nash Equilibrium concept in his PhD thesis at Princeton University. In one of the early scenes of the movie,...
-
Read the case study and answer the question below with a one page response. What does a SWOT analysis reveal about the overall attractiveness of Under Armours situation? Founded in 1996 by former...
-
Leadership in Organizations Module Three Assignment Essay #1: Case Study: Echo Electronics Paul Sanchez is the production manager for Echo Electronics, a small company that makes and distributes...
-
After pollen lands on a stigma, it is far away from the ovule with the megagametophyte, which holds the egg (the megagamete). How are the two sperm cells transported from the stigma to the egg?
-
A parallel-plate capacitor is constructed from a layer of silicon dioxide of thickness 5 10-6 m between two conducting films. The dielectric constant of silicon dioxide is 3.8 and its dielectric...
-
The figure shows a shaft mounted in bearings at A and D and having pulleys at B and C. The forces shown acting on the pulley surfaces represent the belt tensions. The shaft is to be made of ASTM...
-
8. Under what circumstances would the direction of intercompany inventory transactions not affect the allocation of unrealized profit?
-
According to Salary Wizard, the average base salary for a brand manager in Houston, Texas, is $88,592 and the average base salary for a brand manager in Los Angeles, California, is $97,417 (Salary...
-
A wholesale bank is one that both draws funds from businesses and makes loans primarily to businesses. True False Question 2 (2 points)
-
Lewis Company reports the following fixed budget and actual results for May. Prepare a flexible budget performance report showing variances between budgeted and actual results. (Indicate the effect...
-
A solution consisting of a molecular substance of mass 1.14 g dissolved in 100. g of camphor freezes at 176.9C. What is the molar mass of the substance?
-
Nitric oxide, NO, is an intermediate in the production of nitric acid. It is produced commercially by the controlled oxidation of ammonia. Suppose you are considering how to increase the amount of NO...
-
Calculate the integral, assuming that S's f(x) dx = 1, S f(x) dx = 4, Sfe f(x) dx = 7
-
What is the length of the partial wavelength for electromagnetic energy with a frequency of 15 MHz and a phase shift of 263 degrees (in meters)?
-
Let y = f(x) be a function that is differentiable at all real numbers. Suppose f has the form f(x) = {; [cx +6 4+2c for x 1 for x > 1. where b and c are some constants. What is the value of the...
-
Consider the sequence (n) defined by xn = (a) Show that 0In - n! nn (b) Use the result of part (a) and the Squeeze theorem to show that In 0 and n o.
-
Officials of Gwinnett County, one of the fastest growing counties in the country, are looking for ways to expand their sewer system. They are considering two alternative sewer designs. All annual...
-
On August 1, 20Y7, Rafael Masey established Planet Realty, which completed the following transactions during the month: Rafael Masey transferred cash from a personal bank account to an account to be...
-
a) How can disk arrays ensure data reliability and availability? b) Explain RAID 0. c) Explain RAID 1. d) Explain RAID 5.
-
Suppose that you could invest in the following projects but have only $30,000 to invest. How would you make your decision and which projects would you invest in? Project Cost $ 8,000 11,000 9,000...
-
Propose a plausible mechanism for each of the following hydrolysis reactions: (a) (b) (c) (d) EtO OEt * * + 2 ELOH (b) N. * .N'
-
Propose a plausible mechanism for the reaction below: [H2SO4] N- - . -N.
-
As shown above, methenamine is hydrolyzed in aqueous acid to produce formaldehyde and ammonia. Draw a mechanism showing formation of one molecule of formaldehyde (the remaining five molecules of...
-
Given the following financial data for the Smith Corporation, calculate the length of the firm's operating cycle (OC). Sales $2,610,000 Cost of Good Sold $2,088,000 Inventory $ 278,400 Accounts...
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...

Study smarter with the SolutionInn App


