(a) From the graph of Maxwell distribution of speeds in Fig. 3D.6, estimate the most probable speed...
Question:
(a) From the graph of Maxwell distribution of speeds in Fig. 3D.6, estimate the most probable speed of the molecules for each molar mass.
(b) What happens to the fraction of molecules having a speed in the narrow range Δv centered on the most probable speed, vmp, as the molar mass of the gas increases (Eq. 8)?
Fig. 3D.6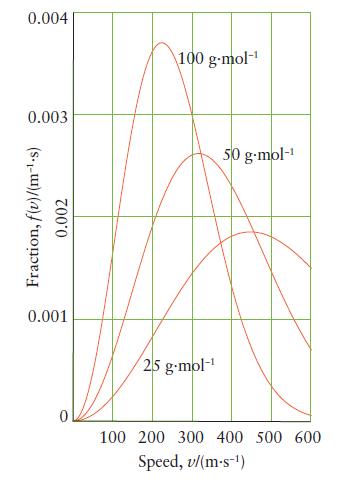
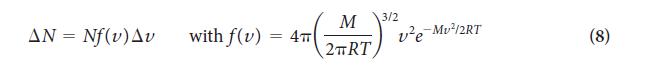
Transcribed Image Text:
0.004 0.003 Fraction, f(u)/(m-.s) 0.002 0.001 100 g-mol- 25 g-mol- 50 g.mol- 100 200 300 400 500 600 Speed, v/(m-s-)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a The most probable speed for the molecule with molar mass of 25 g mol is at ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
(a) From the graph of Maxwell distribution of speeds in Fig. 3D.6, find the location that represents the most probable speed of the molecules at each temperature. (b) What happens to the fraction of...
-
The population of a culture of yeast cells is studied in the laboratory to see the effects of limited resources (food, space) on population growth. At 2-h intervals, the size of the population...
-
A vessel contains 1.00 X 104 oxygen molecules at 500 K. (a) Make an accurate graph of the MaxwellBoltzmann speed distribution function versus speed with points at speed intervals of 100 m/s. (b)...
-
Income statements for the current year and one year ago follow. Assume that all sales are on credit. For Year Ended December 31 Sales Cost of goods sold Other operating expenses Interest expense...
-
The tool is used to shut off gas valves that are difficult to access. If the force F is applied to the handle, determine the component of the moment created about the z axis of thevalve. F=1-601 +...
-
Refer to Figure 18.23. Given L 1 = 3 m, L 2 = 6 m, l 1 = 1.5 m, = 16.5 kN/m 3 , sat = 19.0 kN/m 3 , and ' = 35. a. Find the required depth of the sheet pile, increasing the theoretical estimate by...
-
Understand the advantages and implications of regional integration. LO.1
-
Montana Matt's Golf Inc. was formed on July 1, 2016, when Matt Magilke purchased the Old Master Golf Company. Old Master provides video golf instruction at kiosks in shopping malls. Magilke plans to...
-
did some not sure if its correct need help on the rest. Exercise 17-5 Assigning costs using ABC LO P3 Xie Company identified the following activities, costs, and activity drivers for this year. The...
-
What percentage of space is occupied by close-packed cylinders of length l and radius r?
-
A sample of chlorine gas of volume 1.00 L at 1.00 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. There is a single gaseous...
-
If the difference between tight and slack side tensions for a leather belt does not exceed \(100 \mathrm{~N} / \mathrm{cm}\) of width for a belt \(5 \mathrm{~mm}\) thick, find the maximum stress in...
-
Find the best predicted tip for a ride that is 3.10 miles. How does the result compare to the actual tip of $4.55? Find the best predicted fare amount for a distance of 3.10 miles. How does the...
-
Since the SUTA rates changes are made at the end of each year, the available 2022 rates were used for FUTA and SUTA. Note: For this textbook edition the rate 0.6% was used for the net FUTA tax rate...
-
I have asked three questions with my textbook chapter in which they come from. it would be greatly appreciated if you could help me answer these three questions. Thanks so much. :) 1. What is the...
-
From your reading in Stevens & Smith (2010), a basic description of a medical detoxification, dual-diagnosis inpatient hospital, independent rehab programs, partial hospitalization, halfway houses,...
-
The time between high and low tide in a river harbor is approximately 7 h. The high-tide depth of 16 ft occurs at noon and the average harbor depth is 11 ft. a. Write an equation modeling this...
-
What is the purpose of the journal wizard?
-
Which of the following sets of quantum numbers are not allowed? For each incorrect set, state why it is incorrect. a. n = 3, = 3, m = 0, ms = - 1/2 b. n = 4, = 3, m = 2, ms = - 1/2 c. n = 4, = 1,...
-
From the diagrams of 2p and 3p orbitals in Fig.19 and Fig.20, respectively, draw a rough graph of the square of the wave function for these orbitals in the direction of one of the lobes. Figure 12.19...
-
The wave function for the 2pz orbital in the hydrogen atom is Where a0 is the value for the radius of the first Bohr orbit in meters (5.29 Ã 10-11), Ï is Zr/a0, r is the value for the...
-
Comfort Golf Products is considering whether to upgrade its equipment Managers are considering two options. Equipment manufactured by Stenback Inc. costs $1,000,000 and will last five years and have...
-
Weaver Corporation had the following stock issued and outstanding at January 1, Year 1: 71,000 shares of $10 par common stock. 8,500 shares of $60 par, 6 percent, noncumulative preferred stock. On...
-
Read the following case and then answer questions On 1 January 2016 a company purchased a machine at a cost of $3,000. Its useful life is estimated to be 10 years and then it has a residual value of...

Study smarter with the SolutionInn App


