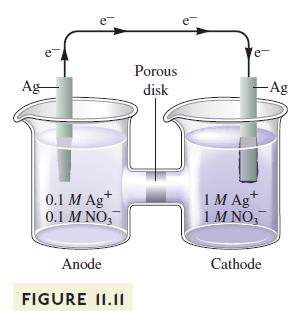
A silver concentration cell similar to the one shown in Fig. 11.11 is set up at 25C
Question:
A silver concentration cell similar to the one shown in Fig. 11.11 is set up at 25°C with 1.0 M AgNO3 in the left compartment and 1.0 M NaCl along with excess AgCl(s) in the right compartment. The measured cell potential is 0.58 V. Calculate the Ksp value for AgCl at 25°C.
Transcribed Image Text:
Ag e 0.1 M Ag+ 0.1 M NO3 Anode FIGURE II.II Porous disk e + 1 M Ag 1M NO, Cathode -Ag
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
In this case at 25C logAg Thus 00591 1 8 058 V 0 wh...View the full answer

Answered By

Jeff Omollo
As an educator I have had the opportunity to work with students of all ages and backgrounds. Throughout my career, I have developed a teaching style that encourages student engagement and promotes active learning. My education and tutoring skills has enabled me to empower students to become lifelong learners.
5.00+
5+ Reviews
52+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Silas is farsighted and wants to read the newspaper. Withoutglasses, Silass near point (the closest point he can see in focus)is far away, so he has to try to read the paper by holding his...
-
Use Gamemaker Studio. Give me a zip file. Create sprites for the player plane and for the background Right click on Sprite and select Create sprite in the resourcepanel. rename the sprite spr_plane...
-
One particular plug-in power supply for a radio looks similar to the one shown in Figure 33.23 and is marked with the following information: Input 120 V AC 8 W Output 9 V DC 300 mA. Assume that these...
-
Show by approximating with integrals that the number of distinct triples of integers between 0 and \(n\) is about \(n^{3} / 6\).
-
A potato of mass 0.5 kg moves under Earths gravity with an air resistive force of kmv. (a) Find the terminal velocity if the potato is released from rest and k = 0.01 s1. (b) Find the maximum height...
-
Two products are manufactured on two sequential machines. The following table gives the machining times in minutes per unit for two products. The daily production quotas for the two products are 80...
-
1 4 Explain accurately to another person Hofstedes four dimensions of national cultures. Evaluate his conclusions on the basis of discussions with your colleagues from any of the countries in his...
-
Benedict Company leased equipment to Mark Inc. on January 1, 2017. The lease is for an eight-year period, expiring December 31, 2024. The first of eight equal annual payments of $600,000 was made on...
-
Dana, who around the city is generally referred to simply as "The Dane," runs an illegal gambling joint. Here are her income and expenses. Calculate her net taxable income from the gambling joint....
-
The first ionization energy for magnesium is \(735 \mathrm{~kJ} / \mathrm{mol}\). Which electron is this for? Estimate \(Z_{\text {eff }}\) for this electron, and explain your reasoning. Calculate...
-
Determine the direction of electron flow, designate the anode and cathode, and calculate the potential at 25C for the cell represented in Fig. 11.12. Fe- 0.01 M Fe+ FIGURE 11.12 Porous disk -Fe 0.1 M...
-
Because diversification is a desirable strategy for avoiding risk, it never makes sense for a bank to specialize in making specific types of loans. Is this statement true, false, or uncertain?...
-
The State of Confusion Legislature passes the following statute: "The State Health Commissioner, when in their opinion, there is sufficient covid - 1 9 vaccine that has been approved by the Federal...
-
Case 1 Baum Co. has two processing departments: Fabrication and Assembly. In the Fabrication Department, metal is cut and formed into various components, which are then transferred to Assembly. The...
-
Your earlier Personal Leadership Assessment, you looked at two areas of your leadership experience, those who led you and those you led. You will again address these two items in your Personal...
-
What is required in this situation: Content slides explaining the qualitative and quantitative steps necessary in conducting a sensitivity analysis. How can a project's risk be incorporated into a...
-
What is "marketing"? What is the difference between "marketing" and the "marketing process"?is it different in your home country vs. North america? Q2. What is the difference between "demand",...
-
Consider a steam power plant that operates on the ideal reheat Rankine cycle. The plant maintains the boiler at 5000 kPa, the reheat section at 1200 kPa, and the condenser at 20 kPa. The mixture...
-
Cassandra Casey operates the Futuristic Antique Store. She maintains subsidiary ledgers for accounts payable and accounts receivable. She presents you with the following information for October 2019:...
-
(a) Calculate the pH of 8.50 * 10 5 m and 7.37 * 10 6 m HCN(aq), ignoring the effect of the autoprotolysis of water. (b) Repeat the calculations, taking into account the autoprotolysis of water.
-
Find the initial concentration of the weak acid or base in each of the following aqueous solutions: (a) A solution of HClO with pH = 5.4; (b) A solution of pyridine, C 5 H 5 N, with pH = 8.8.
-
For the exercise in this topic, base your answers on the potentials listed in Table 6M.1 or Appendix 2B, with the exception of the reduction and oxidation of water at pH = 7: (a) What current is...
-
A contractor constructed a house for resale, which was sold immediately. For tax purposes, this is an example of A) capital income. B) business income. C) other income. D) property income.
-
You invest $100 in a risky asset with an expected rate of return of 0.12 and a standard deviation of 0.15 and a T-bill with a rate of return of 0.05. What percentages of your money must be invested...
-
Nanometrics, Inc., has a beta of 3.43. If the market return is expected to be 13.50 percent and the risk-free rate is 7.00 percent, what is Nanometrics required return? (Round your answer to 2...

Study smarter with the SolutionInn App


