(a) The standard potential of the reduction of Ag 2 CrO 4 to Ag(s) and chromate ions...
Question:
(a) The standard potential of the reduction of Ag2CrO4 to Ag(s) and chromate ions is 10.446 V. Write the balanced halfreaction for the reduction of silver chromate.
(b) Using the data from part(a) and Appendix 2B, calculate the solubility product of Ag2CrO4(s).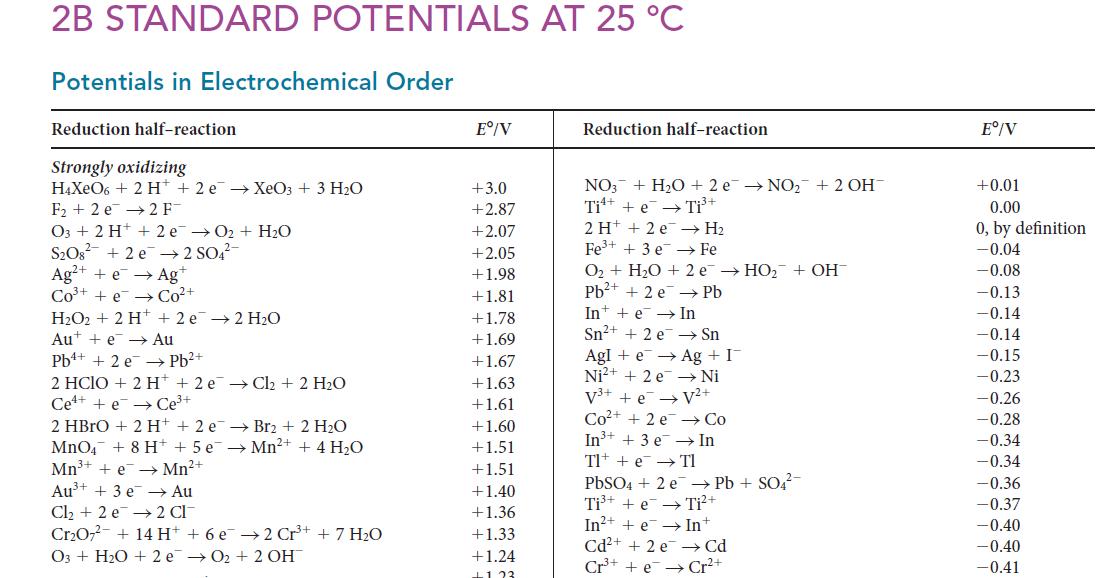
Transcribed Image Text:
2B STANDARD POTENTIALS AT 25 °C Potentials in Electrochemical Order Reduction half-reaction Strongly oxidizing H4XeO6 + 2 H+2 e XeO3 + 3 H₂O F₂2 e 2 F O3 + 2 H+ 2e →0₂ + H₂O S₂O8² +2 e 2 SO4²- Ag+ + e → Agt CO³+ +e Co²+ H₂O2 + 2 H+ + 2e → 2H₂O Aue Au Pb²+ Pb+ + 2 e 2 HClO + 2 H +2 e Cl₂ + 2 H₂O Cee Ce³+ 2 HBrO + 2 H+2 e → Br2₂ + 2 H₂O MnO4 + 8 H+ + 5 e¯ →Mn²+ + 4 H₂O Mn³+ + e→ Mn²+ Au³+ + 3e →→ Au Cl₂ +2 e 2 CI Cr₂O7² + 14 H+ + 6 e 03 + H₂O + 2 e 2 Cr³+ + 7 H₂O 0₂ + 2 OH™ Eº/V +3.0 +2.87 +2.07 +2.05 +1.98 +1.81 +1.78 +1.69 +1.67 +1.63 +1.61 +1.60 +1.51 +1.51 +1.40 +1.36 +1.33 +1.24 +123 Reduction half-reaction NO3 + H₂O +2 e → NO₂ + 2 OH™ Ti + e →→ Ti³+ 2 H+ 2 e → H₂ Fe³+ + 3 e Fe O₂+ H₂O + 2 e → HO₂ + OH Pb²+ + 2e → Pb Int + e → In Sn²+ + 2 e Sn Agle → Ag + I Ni²+ + 2e → Ni V³+ + e → V²+ Co²+ +2e → Co In In³+ + 3 e Tl + e → TI PbSO4 + 2e Ti³+ + e In²+ + e→→ Int Cd²+ +2 e Cd Cr³+ + e→Cr²+ → Pb + SO4²- Ti²+ Eº/V +0.01 0.00 0, by definition -0.04 -0.08 -0.13 -0.14 -0.14 -0.15 -0.23 -0.26 -0.28 -0.34 -0.34 -0.36 -0.37 -0.40 -0.40 -0.41
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The balanced halfreaction for the reduction of silver chromate is Ag2CrO4s 8H 6e 2Ags CrO42 4H2O The ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The Manning Company has financial statements as shown next, which are representative of the company's historical average. The firm is expecting a 35 percent increase in sales next year, and...
-
The standard potential of the Zn2+/ Zn electrode is -0.76 V at 25e. The exchange current density for H+ discharge at platinum is 0.79 mA cm-2 Can zinc be plated on to platinum at that temperature?...
-
The exchange current density for a PtIFe3+,Fel+ electrode is 2.5 mA cm-I. The standard potential of the electrode is +0.77 V. Calculate the current flowing through an electrode of surface area 1.0...
-
9. Let x = (1.11... 111000...) 26, in which the fractional part has 26 1's followed by O's. For the Marc-32, determine x, x+, f(x), x-xx-x, xx, and lx-fl(x)/x.
-
Process further or sell, byproduct. (CMA, adapted) Newcastle Mining Company (NMC) mines coal, puts it through a one-step crushing process, and loads the bulk raw coal onto river barges for shipment...
-
Use Armstrongs axioms to prove the soundness of the decomposition rule.
-
Investigate how the outlier affects the mean and median by doing the following: a. Find the mean score and the median score, with and without the outlier. b. State which measure, the mean or the...
-
Given the following information concerning four stocks, a) Construct a simple price-weighted average, a value-weighted average, and a geometric average. b) What is the percentage increase in each...
-
ui Operating Activity Operating and Investing Activity Not Reported on Cash Flows Financing Activity Noncash Investing and Financing Activity Investing Activity Investing and Financing Activity...
-
Biff rented a pretzel stand and sold pretzels at the beach yesterday. He sold 200 pretzels for $4 each. Here is a list of all of Biff's costs: Pretzel stand rental fee: $80 Wholesale food costs: $240...
-
Calculate the molar concentrations of H 2 SO 3 , HSO 3 , SO 3 2 , H 3 O + , and OH present in 0.170 m Na 2 SO 3 (aq).
-
Suppose that each of the following pairs of redox couples is combined to form a galvanic cell that generates a current under standard conditions. Identify the oxidizing agent and the reducing agent,...
-
What is included as an experts work product?
-
A company determines that monthly sales S(t), in thousands of dollars, after t months of marketing a product is given by S(t) = 23-551 + 230t+ 160. a) Find S'(1), S'(2), and S'(4). b) Find S''(1),...
-
Dan is a 16 year-old who decided to skip his adolescent development class. If Dan was 19 years-old, this would be his choice, but because of his age, he has broken the law. What type of offence did...
-
You need to remove a bolt from a metal door. The maximum torque the bolt can withstand before starting to rotate is 7 = 70 N-m. You apply a wrench of m = 0.5 kg and 1 = 0.3 m long. You push down on...
-
Lesson 10.1: Emotional Intelligence Emotional Intelligence is a type of social intelligence that affords the individual the ability to monitor his own and others' emotions, to discriminate among...
-
Harriet??s annuity has a total cash value of $2000, and she has paid a total of $1,500 into it. Under a Section 1035 exchange, Harriet rolls the entire value of the annuity into a different annuity....
-
In a study of the effect of aluminum intake on the mental development of infants, a group of 92 infants who had been born prematurely were given a special aluminum-depleted intravenous-feeding...
-
Draw and label the E and Z isomers for each of the following compounds: 1. CH3CH2CH==CHCH3 2. 3. 4. CH,CH2C CHCH2CH Cl CH3CH2CH2CH2 CH CH2CCCH2CI CHCH3 CH3 HOCH CH CCC CH O-CH C(CH
-
Show that T = 1 + T ( In z/T) P , and that Pk = 1 P( In z/T) T.
-
A sample containing 42.1 g of Ar is enclosed in a container of volume 0.0885 L at 375 K. Calculate P using the ideal gas, van der Waals, and RedlichKwong equations of state. Based on your results,...
-
The experimental critical constants of CH 4 are found in Table 7.2. Use the values of Pc and T c to calculate V c . Assume that CH 4 behaves as a. An ideal gas b. A van der Waals gas c. A...
-
Hrubec Products, Incorporated, operates a Pulp Division that manufactures wood pulp for use in the production of various paper goods. Revenue and costs associated with a ton of pulp follow: Selling...
-
The AICPA guidelines suggest that taxes should be transparent and visible. This means that: a. The taxes affect similarly situated taxpayers in a similar manner. b. Taxes should be due at the same...
-
What is Apple Companys strategy for success in the marketplace? Does the company rely primarily on customer intimacy, operational excellence, or product leadership? What evidence supports your...

Study smarter with the SolutionInn App


