(a) Using values in Appendix 2A, calculate the standard Gibbs free energy for the vaporization of water...
Question:
(a) Using values in Appendix 2A, calculate the standard Gibbs free energy for the vaporization of water at 25.0°C, 100.0°C, and 150.0°C.
(b) What should the value at 100.0°C be?
(c) Why is there a discrepancy?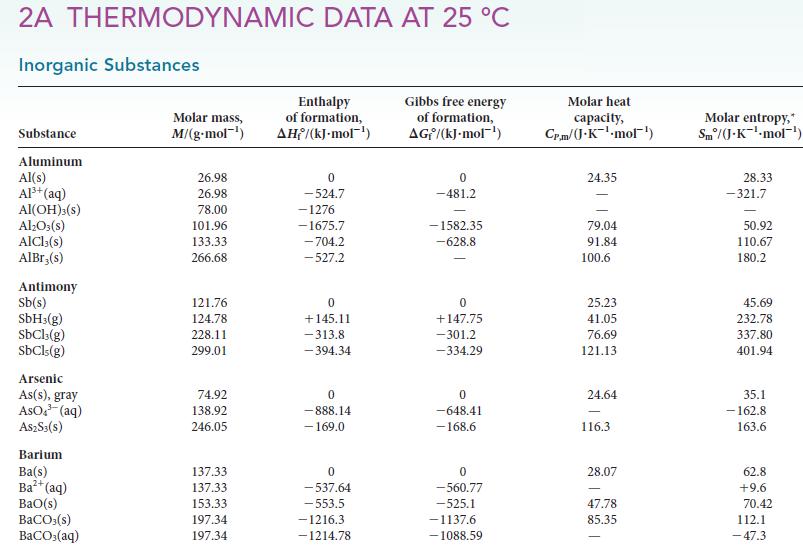
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray ASO³ (aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K-¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J.K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a 857 kJ at 298 K 035 kJ at 373 K 629 ...View the full answer

Answered By

Danish Sohail
My objective is to become most reliable expert for clients. For last 10 years I have been associated with the field of accounting and finance. My aim is to strive for best results and pay particular attention to client needs. I am always enthusiastic to help clients for issues and concerns related to business studies. I can work on analysis of the financial statements, calculate different ratios and analysis of ratios. I can critically evaluate stock prices based on the financial analysis and valuation for companies using financial statements of the business entity being valued with use of excel tools. I have expertise to provide effective and reliable help for projects in corporate finance, equity investments, financial accounting, cost accounting, financial planning, business plans, marketing plans, performance measurement, budgeting, economic research, risk assessment, risk management, derivatives, fixed income investments, taxation, auditing, and financial performance analysis.
4.80+
78+ Reviews
112+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
(a) What is the standard Gibbs free energy of the reaction CO(g) + H 2 O(g) CO 2 (g) + H 2 (g) when K = 1.00? (b) From data available in Appendix 2A, estimate the temperature at which K = 1.00. (c)...
-
The government expects to receive $2.5 million dollars in three years. The government needs the money now so it sells the rights to a third party who charges 12%. How much does the government receive?
-
Using data from Appendix C, calculate the change in Gibbs free energy for each of the following reactions. In each case indicate whether the reaction is spontaneous at 298 K under standard...
-
What line of code can you add to disable all logging messages in your program?
-
Suppose the Geiger tube of Problem 45 is filled with a gas of dielectric constant = 1.8 and breakdown field of 2 106 V/m. (a) What is the maximum potential difference that can be maintained between...
-
Use superposition to find V0 in the network shown. 1 162 42.0"A
-
1. The separate incomes of Pop Corporation and Son Corporation, a 100 percentowned subsidiary of Pop, for 2017 are $2,000 and $1,000, respectively. Pop sells all of its output to Son at 150 percent...
-
Caramel Corporation has 5,000 shares of stock outstanding. In a qualifying stock redemption, Caramel distributes $145,000 in exchange for 1,000 of its shares. At the time of the redemption, Caramel...
-
Businesses operating in highly competitive environments are more likely to undertake investments that are not profitable
-
In the model displayed in the Exhibit above, which predictor variables are most likely to be chosen by a Stepwise regression approach for best model fit. You may use JMP Stepwise Regression operation...
-
Given that the atomic orbitals used to form hybrids are normalized to 1 and mutually orthogonal, (a) Show that the two tetrahedral hybrids h 1 = s + p x + p y + p z and h 3 = s - p x = p y - p z are...
-
Determine the payment required to settle the invoice on the indicated payment date.
-
Major League Baseball Rule 1.09 states that "the baseball shall weigh not less than=or more than=14 ounces" (www.mlb.com). Use these values as the lower and the upper control limits, respectively....
-
Describe in your own words how you would expect the data points on a scatterplot to be distributed if the following features were present (i.e. for each part, explain how the feature would look on a...
-
imagine this experimental setup: One temperature probe is in embedded in a small block of frozen sugar water at -20. The frozen sugar water is in a small test tube The melting/freezing point of this...
-
Question 2: (40 points: 10 each) During September, Sweet Foods manufactures a single product. The Company's material purchases amounted to 9,000 pounds at a price of $9.80 per pound. Actual costs...
-
E12-23 (Algo) (Supplement 12B) Preparing a Statement of Cash Flows, Indirect Method: T-Account Approach [LO 12-S2] Golf Goods Incorporated is a regional and online golf equipment retailer. The...
-
A symmetric compound channel in over bank flow has a main channel with a bottom width of 30 m, side slopes of 1:1, and a flow depth of 3m. The floodplains on either side of the main channel are both...
-
a) What is the difference between data and information? b) How can data be protected while it is being transmitted? c) How can data be protected while it is being processed? d) What are some ways...
-
Which should drive action planning more, strengths or weaknesses? That is, is it more important to build on your strengths or to reduce your weaknesses? Explain.
-
Draw a plausible mechanism for each of the following transformations: (a) (b) (c) (d) (e) (f) CHs `H + H3C H H30* OCH3 H. ,
-
Each transformation below shows a starting material being converted into a product (the reagents necessary to achieve the transformation have not been shown). For each transformation, determine...
-
Identify whether each transformation below involves an increase, a decrease, or no change in the number of hydrogen atoms: a. b.
-
How do warehouses and distribution centers differ? What is cross-docking and why might a company choose to cross-dock a product? What kinds of products can be delivered electronically? What kinds...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...
-
Guzman company received a 60- day, 5 % note for 54,000 dated July 12 from a customer on account. Determine the due date on note. Determine the maturity value of the note and journalize the entry of...

Study smarter with the SolutionInn App


