Because the polarity of a molecule affects its physical properties, you need to know whether a molecule
Question:
Because the polarity of a molecule affects its physical properties, you need to know whether a molecule is polar or nonpolar when predicting how it might interact with other molecules. Predict whether
(a) A boron trifluoride molecule, BF3, and
(b) An ozone molecule, O3, are polar.
ANTICIPATE The bonds in BF3 are very polar, but the bonds in O3 are not, so you might expect BF3 to be polar and O3 to be nonpolar. However, the shape of a molecule determines the polarity of the molecule, so the shapes must be identified before you can make a prediction.
PLAN In each case, predict the shape of the molecule by using the VSEPR model and then decide whether the dipole moments associated with the bonds cancel as a result of the symmetry of the molecule. If necessary, refer to Fig. 2E.7.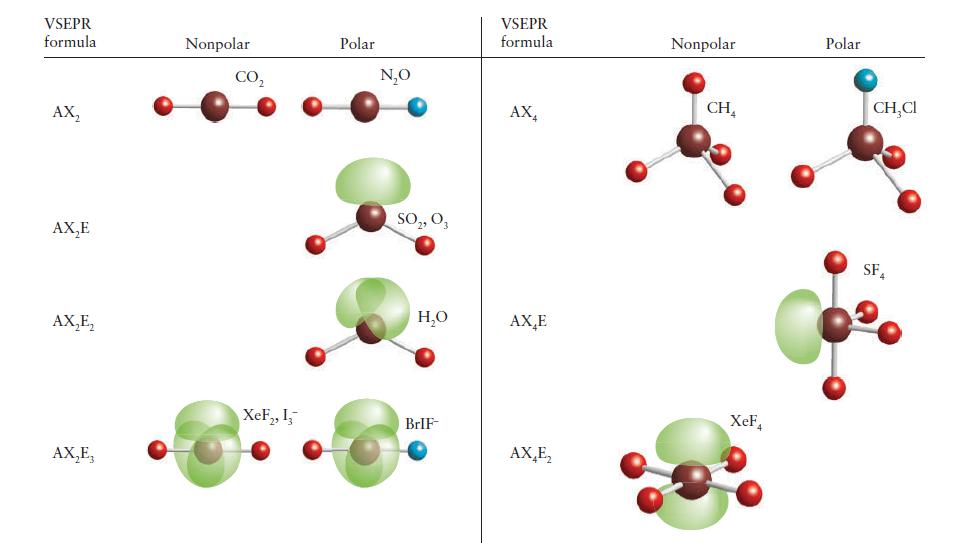
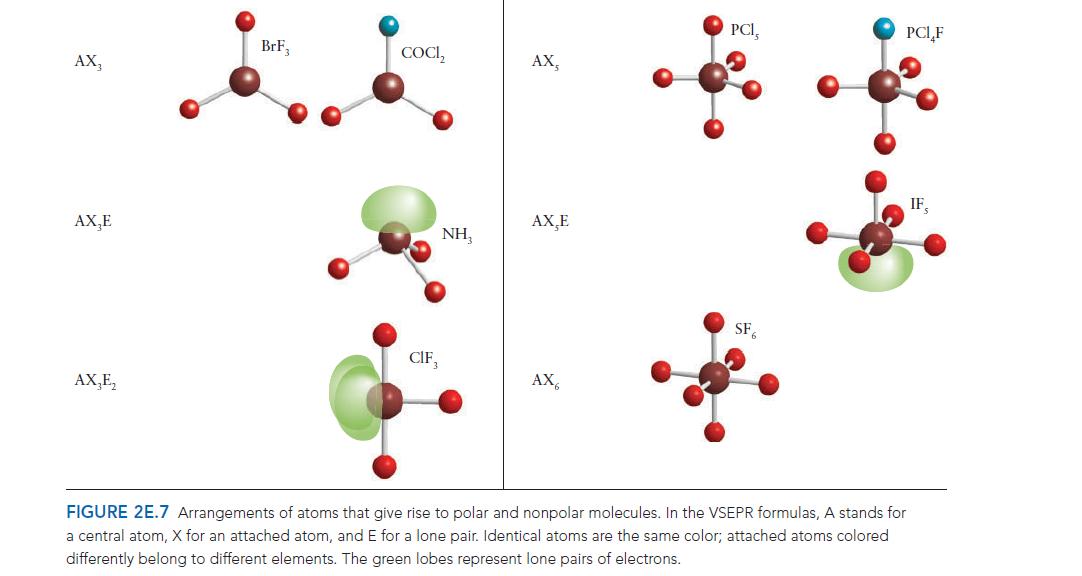
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





