Below are two absorption plots. One was obtained from a solution of an organic dye and the
Question:
Below are two absorption plots. One was obtained from a solution of an organic dye and the other from a quantum dot suspension. Which plot was obtained from which solution? Explain your reasoning.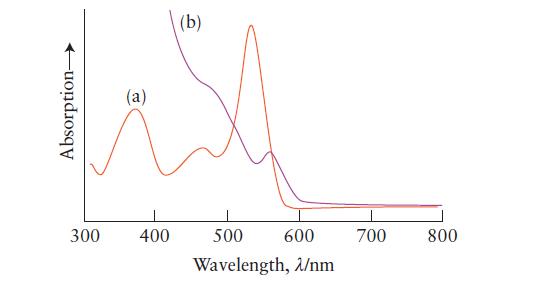
Transcribed Image Text:
Absorption →→ (a) 300 400 (b) 500 600 Wavelength, 2/nm 700 800
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Plot a Was obtaine...View the full answer

Answered By

Elias Gichuru
am devoted to my work and dedicated in helping my clients accomplish their goals and objectives,providing the best for all tasks assigned to me as a freelancer,providing high quality work that yields high scores.promise to serve them earnestly and help them achieve their goals.i have the needed expertise,knowledge and experience to handle their tasks.
4.80+
325+ Reviews
859+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
A dilute aqueous solution of an organic compound soluble in water is formed by dissolving 2.35 g of the compound in water to form 0.250 L of solution. The resulting solution has an osmotic pressure...
-
The types of raw materials used to construct stone tools found at the archaeological site Casa del Rito are shown below (Bandelier Archaeological Excavation Project, edited by Kohler and Root). A...
-
The following off-resonance-decoupled carbon NMR was obtained from a compound of formula C3H5Cl3. Propose a structure for this compound, and show which carbon atoms give rise to which peaks in the...
-
Given the following set of slope staking notes: C Sta 51+00 50+00 49+00 L CX 33.4 F 9.1 33.6. F 10.3 35.4 C 5.5 0.0 F 3.5 R C 3.2 X C 4.1 30.2 0.0 20.0 Bases Base for cut=48 ft Base for fill= 40 ft s...
-
The distributed load acts on the beam as shown. Determine the maximum intensity wmax. What is the magnitude of the equivalent resultant force? Specify where it acts, measured from pointB. w= (-2x +...
-
Suppose that instead of maximizing hits per minute, constraints, a web server company wants to minimize cost while maintaining a rack of standard and cuttingedge servers that can handle at least...
-
4. Using the information in the previous problem, compute the prices of a. An Asian arithmetic average strike call. b. An Asian geometric average strike call.
-
The Byers Company presents the following condensed income statement for 2007 and condensed December 31, 2007 balance sheet: Additional information: 1. The company's common stock and preferred stock...
-
Splish Brothers Inc. has been manufacturing its own finials for its curtain rods. The company is currently operating at 100% of capacity, and variable manufacturing overhead is charged to production...
-
What is the total mass of (a) The electrons; (b) The (nuclear) protons in a block of the ceramic BaTiO 3 of mass 4.72 kg?
-
Draw a simple chemical picture to show how the removal of water helps to make aluminosilicates into rigid ceramics.
-
On December 21, 2012, Zurich Company provided you with the following information regarding its trading securities. During 2013, Carolina Company stock was sold for $9,500. The fair value of the stock...
-
Recognition is a very important element of volunteer management. Do you know someone who has done amazing volunteer work for a good cause? Wouldn't it be nice to thank them with an award! Take a look...
-
What do Financial Planners do? Would you consider hiring a Financial Planner? How important are ethics when working with a financial planning professional? Explain the concept of return on...
-
Explain the specific perceptual errors you made of EACH of your teammates during the class exercise
-
Identify the company that makes the product a. Are they a large company or a small company? b. Are they a chain or a major corporation? c. Have they been around for decades or are they a new company?...
-
The Red Inn at Pismo is a 150-room hotel that caters mainly to business clients during the week and to tourists during the weekends and in the summer. Below is a table summarizing the average daily...
-
Develop the parametric equations of a hyperbola by following these steps. a. Write the equation of a unit hyperbola in standard form using x and y. b. Replace x with 1 / cos t and solve for y in...
-
A container holds 2.0 mol of gas. The total average kinetic energy of the gas molecules in the container is equal to the kinetic energy of an 8.0 10-3-kg bullet with a speed of 770 m/s. What is the...
-
Consider the following data for three binary compounds of hydrogen and nitrogen: When 1.00 L of each gaseous compound is decomposed to its elements, the following volumes of H2(g) and N2(g) are...
-
A 0.200- g sample of protactinium(IV) oxide is converted to another oxide of protactinium by heating in the presence of oxygen to give 0.2081 g of the new oxide, PaxOy. Determine the values of x and...
-
A 1.000- g sample of XI 2 is dissolved in water, and excess silver nitrate is added to precipitate all of the iodide as AgI. The mass of the dry AgI is found to be 1.375 g. Calculate the atomic...
-
Create a Data Table to depict the future value when you vary the interest rate and the investment amount. Use the following assumptions: Interest Rates: Investment Amounts:-10.0% $10,000.00 -8.0%...
-
Isaac earns a base salary of $1250 per month and a graduated commission of 0.4% on the first $100,000 of sales, and 0.5% on sales over $100,000. Last month, Isaac's gross salary was $2025. What were...
-
Calculate the price, including both GST and PST, that an individual will pay for a car sold for $26,995.00 in Manitoba. (Assume GST = 5% and PST = 8%) a$29,154.60 b$30,234.40 c$30,504.35 d$28,334.75...

Study smarter with the SolutionInn App


