Calculate the equilibrium constant at 25C for each of the following reactions, by using data in Appendix
Question:
Calculate the equilibrium constant at 25°C for each of the following reactions, by using data in Appendix 2A: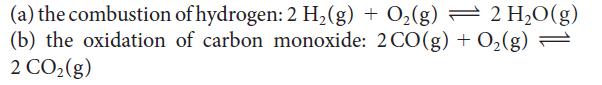
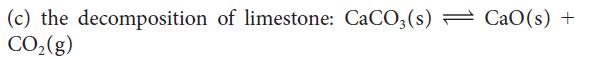
Transcribed Image Text:
(a) the combustion (b) the oxidation 2 CO₂(g) of hydrogen: 2 H₂(g) + O₂(g) — 2 H₂O(g) of carbon monoxide: 2 CO(g) + O₂(g) =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a K 1 10 ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard enthalpy, entropy, and Gibbs free energy at 298 K for each of the following reactions by using data in Appendix 2A. For each case, confirm that the value obtained from the...
-
The budgeted income statement for Barnaby's Hideaway is produced on your Excel spreadsheet. Assume that the following constitute the fixed and vari- able costs for the upcoming year Fixed Costs for...
-
Case1. 640,000 viewers interested in Sports and 360,000 viewers interested in Economy C2 Sports Economy V3 Sports 32,32 36,64 Economy 64, 36 18,18 Questions: 1. What is the equilibrium of the game?...
-
In Exercises 8586, find a. (f g)(x); b. (g f)(x); c. (f g)(3). f(x)=x, g(x) = x + 1
-
A 0.12-F capacitor is given a charge Q0. After 4 s, its charge is Q0. What is the effective resistance across this capacitor?
-
A position-time graph for a particle moving along the x axis is shown in Figure P2.7. (a) Find the average velocity in the time interval t = 1.50 s to t = 4.00 s (b) Determine the instantaneous...
-
(Earnings Management) Grace Inc. has recently reported steadily increasing income. The company reported income of $20,000 in 2001, $25,000 in 2002, and $30,000 in 2003. A number of market analysts...
-
Two identical spheres are each attached to silk threads of length L = 0.500 m and hung from a common point (Fig. 21.44). Each sphere has mass m = 8.00 g. The radius of each sphere is very small...
-
11.2 Accounting for Stock Issuances How does the purchase of treasury stock affect current shareholders
-
Ayayai Hotels Ltd. (AHL) is a small boutique hotel that provides 44 suites that can be rented by the day, week, or month. Food service is available through room service as well. In addition, there...
-
A reaction mixture that consisted of 0.20 mol N 2 and 0.20 mol H 2 was introduced into a reactor of volume 25.0 L and heated. At equilibrium, 5.0% of the nitrogen gas had reacted. What is the value...
-
Puddings contain large starch molecules that cause the mixture to thicken by a mechanism similar to that by which gelatin thickens. Which of the following suggestions is the best description of how...
-
Under what conditions can firms gain first-mover advantages in an emerging industry?
-
4. What is the time complexity of the following procedure for in/2 to n do j 2 end for while (j
-
If the concentration of a constituent in the influent to the equalization basin is constant over the 24 h period, will the load of the constituent from the basin be constant? If the concentration of...
-
A three-phase transmission line of a 60 Hz circuit has a length of 370 km (230 miles). the conductors are of the 795,000cm (54/7) type with horizontal spacing of 25 feet between them. The load on the...
-
Simulate rolling a dice using Math.random() . Your roll function should allow the caller to specify any number of sides, but default to 6 if no side count is given: roll() assumes a 6 sided dice,...
-
Drama Read the excerpt from a play. Then, answer the question(s). (1) (2) Belle: Having trouble deciding what will make you look like both a power to be reckoned with and a fetching young lady while...
-
An inverted hemispherical shell of diameter d as shown in Figure P2.6.3 is used to cover a tank filled with water at 20°C. Detuning the minimum weight the shell needs to be to hold itself in...
-
Integration is a vital concept when applied in one?s life. Integrating your life means making ideal choices. Perfect choices on the other go in line with quality decisions. Quality decisions lead to...
-
Identify the mechanism expected to operate when 2-bromo-2-methylpentane is treated with each of the following reagents: a) EtOH b) t-BuOK c) NaI d) NaOEt e) NaOH
-
When 1-chlorobutane is treated with ethanol, neither elimination process (E1 or E2) is observed at an appreciable rate; a) Explain why an E2 reaction does not occur. b) Explain why an E1 reaction...
-
Assuming that H o f is constant in the interval 275 K600. K, calculate G o for the process (H 2 O, g, 298 K) (H 2 O, g, 600.K). Calculate the relative change in the Gibbs energy.
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


