Calculate the unknown concentration of the ion in each of the following cells: 2+ (a) Pb(s) Pb+
Question:
Calculate the unknown concentration of the ion in each of the following cells: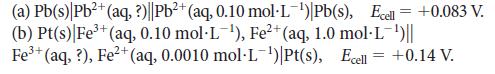
Transcribed Image Text:
2+ (a) Pb(s) Pb²+ (aq, ?)||Pb²+ (aq, 0.10 mol-L-¹) Pb(s), Ecell = +0.083 V. (b) Pt(s)| Fe³+ (aq, 0.10 mol-L-¹), Fe²+ (aq, 1.0 mol-L-¹)|| 2+ Fe³+ (aq, ?), Fe²+ (aq, 0.0010 mol-L-¹)|Pt(s), Ecell = +0.14 V.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a The cell notation can be written as Pbs Pb aq Pb aq 010 mol L Pbs Since the cell potential Ecell i...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The concentration of Fe3+ ion in a sample of H2O is 335.0 ppm. What mass of Fe3+ ion is present in 3,450 mL of H2O, which has a density of 1.00 g/mL?
-
Is the criterion 6 3CO 2 ) 2 (aq) is found to be 9.11. However, the contribution to the pH from the autoprotolysis of water was ignored. Repeat the calculation of the pH of this solution, taking into...
-
Calcium acetate, Ca(CH 3 CO 2 ) 2 (aq), is used to treat patients with a kidney disease that results in high levels of phosphate ions in the blood. The calcium binds to the phosphates so that they...
-
Digital Camera Shop Inc. uses the lower-of-cost-or-market basis for its inventory. The following data are available at December 31. Instructions What amount should be reported on Digital Camera...
-
Payback, even and uneven cash flows. You have the opportunity to expand your business by purchasing new equipment for $159,000. You expect to incur cash fixed costs of $96,000 per year to use this...
-
Define suitable populations from which the following samples are selected: (a) Persons in 200 homes are called by telephone in the city of Richmond and asked to name the candidate that they favor for...
-
List two suggestions for ensuring adequate change control on projects that involve outside contracts. LO.1
-
Scrap, job costing. The Morgan Company has an extensive job-costing facility that uses a variety of metals. Consider each requirement independently. Required 1. Job 372 uses a particular metal alloy...
-
The free cash flow to the firm has been reported as $194 million. The pre-tax interest expense to the firm is $23 million. If the tax rate is 33% and the net debt of the firm increased by $46...
-
Faced with rising pressure for a $15 per hour minimum wage rate, the farming industry is currently exploring the possible use of robotics to replace some farm workers. The Lettuce Bot is one such...
-
Calculate the pH and pOH of (a) A solution that is 0.23 m Na 2 HPO 4 (aq) and 0.18 m Na 3 PO 4 (aq); (b) A solution that is 0.45 m Na 2 HPO 4 (aq) and 0.62 m Na 3 PO 4 (aq); (c) A solution that is...
-
Suppose that 1.773 g of impure barium hydroxide is dissolved in enough water to produce 200. mL of solution and that 25.0 mL of this solution is titrated to the stoichiometric point with 13.1 mL of...
-
Read Don Hummer's "Serious Criminality at U. S. College and Universities: An Application of the Situational Perspective" (2004) via Library Electronic Reserves. Please assess the validity of the...
-
Maria A Solo (SSN 318-01-6921) lives at 190 Glenn drive, grand rapids, Michigan 49527-2005. Maria (age 45 and single) claims her aunt, Selda Ray (ssn 282-61-4011), as a dependent. Selda lives with...
-
A clinical trial was conducted to test the effectiveness of a drug used for treating insomnia in older subjects. After treatment with the drug, 11 subjects had a mean wake time of 95.1 min and a...
-
PROBLEM 13-3 Translation-Local Currency Is the Functional Currency LO7 (This problem is a continuation of the illustration presented in the chapter.) On January 2, 2019, P Company, a US-based...
-
The operations manager for a local bus company wants to decide whether he should purchase a small, medium, or large new bus for his company. He estimates that the annual profits (in $000) will vary...
-
Claim: Fewer than 8.2% of homes have only a landline telephone and no wireless phone. Sample data: A survey by the National Center for Health Statistics showed that among 13,215 homes 5.78% had...
-
The shell of the land snail Limocolaria martensiana has two possible color forms: streaked and pallid. In a certain population of these snails, 60% of the individuals have streaked shells. Suppose...
-
Test whether the 5-year survival rate for breast cancer is significantly different between African American and Caucasian women who are younger than 50 years of age and have localized disease....
-
The racemization process described in the previous problem also occurs in acidic conditions. Draw a mechanism for the racemization process in aqueous acid.
-
Draw all four -hydroxyaldehydes that are formed when a mixture of acetaldehyde and pentanal is treated with aqueous sodium hydroxide.
-
Identify all of the different -hydroxyaldehydes that are formed when a mixture of benzaldehyde and hexanal is treated with aqueous sodium hydroxide.
-
DETAILS 1. [-/1 Points) SMITHNM13 11.2.025. MY NOTES Convert the credit card rate to the APR. Oregon, 2% per month % Need Help? ReadIt Watch
-
Corom Stack Standard CALCULATOR PRINTER VERSION BACK NEXT Problem 13-02A a-c (Part Level Submission) Sheffield Corporation had the following stockholders' equity accounts on January 1, 2020: Common...
-
Suppose that you own 2,100 shares of Nocash Corp. and the company is about to pay a 25% stock dividend. The stock currently sells at $115 per share. a. What will be the number of shares that you hold...

Study smarter with the SolutionInn App


