Complete a Lewis structure for the compound shown below, then answer the following questions. How many carbon
Question:
Complete a Lewis structure for the compound shown below, then answer the following questions. How many carbon atoms are sp2 hybridized? How many CON bonds are formed by the overlap of an sp3 hybridized carbon with an sp3 hybridized nitrogen? How many lone pairs of electrons are in the Lewis structure of your molecule? How many π bonds are present?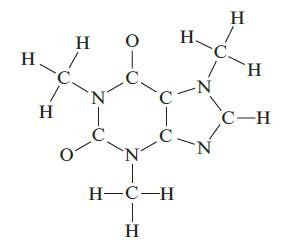
Transcribed Image Text:
H H H N T O 0- C N H-C-H T H H. C. -C -N N H H C-H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
Answer There are four carbon atoms sp2 hybridized There are three CON bonds formed by ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
How many carbon atoms of citronellal would be radioactively labeled if the acetic acid used in the experiment were enriched with 14C at C-1 instead of at C-2? Identify these carbon atoms.
-
How many carbon atoms of citronellal would be radioactively labeled if the acetic acid used in the experiment were enriched with 14C at C-1 instead of at C-2? Identify these carbon atoms.
-
Complete the Lewis structure of the compound shown below and indicate which of the following statements are true. The C-N bond is formed from overlap of an sp 3 hybrid orbital from the carbon atom...
-
Using Figure 7. 4, show how the level of oil imports and the price level would be affected if the country represented in that figure acted to internalize national security issues, but ignored climate...
-
The time spent (in days) waiting for a kidney transplant for people ages 3549 can be approximated by a normal distribution, as shown in the figure. (a) What waiting time represents the 80th...
-
A placement test is given by a university to predict student success in a calculus course. On average, 70% of students who take the test pass it, and 87% of those who pass the test also pass the...
-
Coral reefs How sensitive to changes in water temperature are coral reefs? To find out, measure the growth of corals in aquariums where the water temperature is controlled at different levels. Growth...
-
The first case at the end of this chapter and each of the remaining chapters is a series of integrative cases involving Starbucks. The series of cases applies the concepts and analytical tools...
-
prepaid insurance account had 27,744 debit balance at December 31 before adjusting for the costs of any expired coverage for the year. an analysis of prepaid insurance shows that 20,004 of unexpired...
-
n For the circuit shown in the figure (Figure 1), find the current through and the potential difference across each resistor For the steps and strategies involved in solving a similar problem, you...
-
In which of the following diatomic molecules would the bond strength be expected to weaken as an electron is removed? a. H 2 b. B 2 c. C 2 2- d. OF
-
Ronald Robinson, Wyman Robinson, and Friendly Discount Auto Sales (appellants) appeal from the granting of summary judgment in favor of appellee Mike Durham (Durham). The facts material to this...
-
What is the difference between a directional and a non directional significance test?
-
Steve Reese is a well-known interior designer in Fort Worth, Texas. He wants to start his own business and convinces Rob ODonnell, a local merchant, to contribute the capital to form a partnership....
-
One of the main purposes of evaluation research is: a. reexamining previously collected data. b. monitoring and improving programs. c. generating rich descriptions of individual perspectives. d....
-
10.13 Sweetlip Ltd and Warehou Ltd are two family-owned flax-producing companies in New Zealand. Sweetlip Ltd is owned by the Wood family and the Bradbury family owns Warehou Ltd. The Wood family has...
-
distribution that is skewed to the right instead of being normally distributed. Assume that we collect a random sample of annual incomes of 50 statistics students. Can the distribution of incomes in...
-
Swain Athletic Gear (SAG) operates six retail outlets in a large Midwest city. One is in the center of the city on Cornwall Street and the others are scattered around the perimeter of the city....
-
Find the distance between each pair of points, and give the coordinates of the midpoint of the line segment joining them. P(3, -1), Q(-4, 5)
-
(a) Find the equation of the tangent line to f(x) = x 3 at the point where x = 2. (b) Graph the tangent line and the function on the same axes. If the tangent line is used to estimate values of the...
-
A solution of phosphoric acid was made by dissolving 10.0 g H 3 PO 4 in 100.0 mL water. The resulting volume was 104 mL. Calculate the density, mole fraction, molarity, and molality of the solution....
-
What is ion pairing?
-
Common commercial acids and bases are aqueous solutions with the following properties: Calculate the molarity, molality, and mole fraction of each of the preceding reagents. Hydrochloric acid Nitric...
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...

Study smarter with the SolutionInn App


