Complete and balance each of the following equations: (a) HS(aq) + O(g) (b) CaO (s) +
Question:
Complete and balance each of the following equations: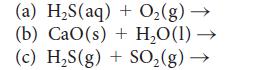
Transcribed Image Text:
(a) H₂S(aq) + O₂(g) → (b) CaO (s) + H₂O(1)→ (c) H₂S(g) + SO₂(g) -
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
a 2 HSg 30g b Ca...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Complete and balance each of the following molecular equations (in aqueous solution); include phase labels. Then, for each, write the net ionic equation. a. NaOH + HNO3 b. HCl + Ba(OH)2 c. HC2H3O2...
-
Complete and balance each of the following molecular equations (in aqueous solution); include phase labels. Then, for each, write the net ionic equation. a. Al(OH)3 + HCl b. HBr + Sr(OH)2 c....
-
Complete and balance each of the following molecular equations, including phase labels, if a reaction occurs. Then write the net ionic equation. If no reaction occurs, write NR after the arrow. a....
-
Seth borrows X for ten years at an annual effective interest rate of 4%, to be repaid with equal payments at the end of each year. The outstanding loan balance at the end of the eighth year is...
-
Consider the following capital rationing problem: Set up this problem as a linear program. NPV Project Co C2 -10,000 -10,000 +6,700 +9,000 -20,000 +5,000 +5,000 +5,000 +5,000 +4,000 -15,000 -1,500...
-
Draw an activity diagram that models the following scenario for a point of sale system. [15] the sales clerk enters item codes until all the customers purchases are recorded the subtotal, taxes and...
-
Particle physicists use the energytime uncertainty relation to estimate the lifetimes of unstable particles produced in high-energy particle accelerators (Chapter 39). Some particles have lifetimes...
-
The financial statements of P&G are presented in Appendix 5B or can be accessed at the books companion website, Instructions Refer to P&Gs financial statements and accompanying notes to answer the...
-
Selected Stock Transactions Diamondback Welding & Fabrication Corporation sells and services pipe welding equipment in Illinois. The following selected accounts appear in the ledger of Diamondback...
-
Complete and balance the following equations: (a) BO3(s) + Mg(1) (b) Al(s) + Cl(g) (c) Al(s) + O(g)
-
Urea, CO(NH 2 ) 2 , reacts with water to form ammonium carbonate. Write the chemical equation and calculate the mass of ammonium carbonate that can be obtained from 4.0 kg of urea.
-
How do static and adaptive forecasting methods differ?
-
Based on the reading,How to make sure your next product or service launch drives growth (click the underlined link),what stands out to you as the most important factor in a differentiated launch...
-
OM in the News has previously looked at the Waffle House Index, used to measure the damage from hurricanes. The index made the news again for Hurricane Ian. According to the Boston Globe, 40 Waffle...
-
Mixture of persuasive and negative formal I am Elizabeth grinderFirst part email Next part setting up the meeting Final part memo The reader is Robert * do not come off accusatory*** Project TWO:...
-
Anyone who has sampled today's social media offerings has probably experienced this situation: You find a few fascinating blogs, a few interesting people to follow on Twitter, a couple of podcast...
-
a. Begin with a converging lens of focal length f. Place an illuminated object a distance p, in front of the lens. For all positive values of p;: 1. calculate and sketch a graph of the location of...
-
Use the data in HTV.RAW for this exercise. (i) Run a simple OLS regression of log{wage) on educ. Without controlling for other factors, what is the 95% confidence interval for the return to another...
-
7 A 29-year-old, previously healthy man suddenly collapses at a party where legal and illicit drugs are being used. Enroute to the hospital, he requires resuscitation with defibrillation to establish...
-
The transition has two lines given by vÌ = 25354.8 cm 1 and vÌ = 25242.7 cm 1 . The transition has three lines given by vÌ = 32444.8 cm 1 , vÌ = 32334.0 cm 1 , and...
-
Calculate the mean value of the radius r at which you would find the electron if the H atom wave function is 210 (r, , ).
-
The total energy eigenvalues for the hydrogen atom are given by E n = e 2 / (8Ïε 0 a 0 n), n = 1, 2, 3, 4,¦, and the three quantum numbers associated with the total energy...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


