Complete and balance each of the following reactions: (a) XeF (s) + HO(1)XeO3(aq) + HF(aq) (b) Pt(s)
Question:
Complete and balance each of the following reactions: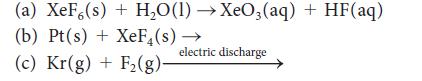
Transcribed Image Text:
(a) XeF (s) + H₂O(1)→XeO3(aq) + HF(aq) (b) Pt(s) + XeF₁(s) (c) Kr(g) + F₂(g)- → electric discharge
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Sure here are the balanced chem...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Complete and balance each of the following molecular equations (in aqueous solution); include phase labels. Then, for each, write the net ionic equation. a. NaOH + HNO3 b. HCl + Ba(OH)2 c. HC2H3O2...
-
Complete and balance each of the following molecular equations (in aqueous solution); include phase labels. Then, for each, write the net ionic equation. a. Al(OH)3 + HCl b. HBr + Sr(OH)2 c....
-
Complete and balance each of the following molecular equations, including phase labels, if a reaction occurs. Then write the net ionic equation. If no reaction occurs, write NR after the arrow. a....
-
Using and citing Bevan, define active empathic listening. Report on both the meaning and significance of the definition Explain how you can use active empathic listening to help you improve your...
-
Here are two useful rules of thumb. The Rule of 72 says that with discrete compounding the time it takes for an investment to double in value is roughly 72/interest rate (in percent). The Rule of 69...
-
Determine the lattice coefficients corresponding to the FIR filter with system function H(z) = A3(z) = 1 + 12/24z 1 + 5/8z 2 + 1/3z 3
-
In this exercise, you modify the application from Exercise 2. Use Windows to make a copy of the Workers Solution folder. Rename the copy Workers Solution-Filename. a. Open the Workers Solution.sln...
-
What is an RFP, and what critical tasks does it facilitate in the purchasing process?
-
Which of the following statements is incorrect as a requirement for a good investment evaluation tool? A. A good investment evaluation tool must consider different levels of risk involved. B. A good...
-
What justification is there for regarding the ammonium ion as an analog of a Group 1 metal cation? Consider properties such as solubility, charge, and radius. The radius of NH 4 + treated as a...
-
The enthalpy of dissociation of hydrogen bonds, H HBond , is a measure of their strength. Explain the trend seen in the data for the following pure substances, which were measured in the gas phase:
-
Suppose two firms in an industry face linear inverse demand curves P i (q i , q j ) = 7- q i -q j , i = 1; 2, i j. Firms compete in a two-stage game; first they set capacity and then they set price...
-
Your friend Amber has approached you seeking advice concerning two investment opportunities that she is presently considering. Her classmate Simone has asked her for a loan of $5,000 to help...
-
Please read the following carefully. For each question on the exam, you should assume that: 1. unless expressly stated to the contrary, all events occurred in ?the current taxable year;? 2. all...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Can I get clear explanation how to work these. Thanking you in advance. 1. A rod 12.0 cm long is uniformly charged and has a total charge of -23.0 uC. Determine the magnitude and direction of the...
-
Poll Results in the Media USA Today provided results from a survey of 1144 Americans who were asked if they approve of Brett Kavanaugh as the choice for Supreme Court justice. 51% of the respondents...
-
Use the data in CPS78_85.RAW for this exercise. (i) How do you interpret the coefficient on y85 in equation (13.2)? Does it have an interesting interpretation? (Be careful here; you must account for...
-
Find the intercepts and then graph the line. (a) 2x - 3y = 6 (b) 10 - 5x = 2y
-
In P24.3, the hybrid bonding orbitals for ozone were derived. Use the framework described in Section 24.3 to derive the normalized hybrid lone pair orbital on the central oxygen in O 3 that is...
-
Using your results from Problem P24.10, a. Calculate the s and p character of the water lone pair hybrid orbitals b. Show that the lone pair orbitals are orthogonal to each other and to the hybrid...
-
Use the VSEPR method to predict the structures of the following: a. PF 3 b. CO 2 c. BrF 5 d. SO 2 3
-
18. Suppose that Maxima shares are selling for $10 per share and you own a call option to buy Maxima shares at $7.50. The intrinsic value of your option is:
-
ABC Insurance Company reported the following information on its accounting statements last year: What was ABC 's expense ratio last year
-
Calculate the current ratio and the quick ratio for the following partial financial statement for Tootsie Roll Note: Round your answers to the nearest hundredth

Study smarter with the SolutionInn App


