Complete the following table (all values are in kilojoules per mole). Compound, , ion , eg MX
Question:
Complete the following table (all values are in kilojoules per mole).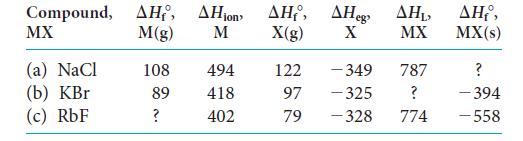
Transcribed Image Text:
Compound, ΔΗ, ΔΗion ΔΗ, ΔΗeg MX M(g) M X(g) X (a) NaCl (b) KBr (c) RbF 108 89 ? 494 418 402 122 97 79 - 349 -325 -328 ΔΗ, MX 787 ? 774 ΔΗ,, MX(s) ? -394 -558
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 412 kJ ...View the full answer

Answered By

Ankur Gupta
I have a degree in finance from a well-renowned university and I have been working in the financial industry for over 10 years now. I have a lot of experience in financial management, and I have been teaching financial management courses at the university level for the past 5 years. I am extremely passionate about helping students learn and understand financial management, and I firmly believe that I have the necessary skills and knowledge to effectively tutor students in this subject.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Compute the amount of the gift in the following independent scenarios. If an amount is zero, enter "0". a. Bolivar and Collen purchase real estate as tenants in common. Of the $250,000 purchase...
-
Which of the following would produce a materials price variance? abreakage of materials in production. ban excess quantity of materials used can excess number of direct labor-hours worked in...
-
Calculate a lattice energy for CaH2(s) in kilojoules per mole using the following information: (a) Eea for H(g) = -72.8kj/mol. (b) Ei1 for Ca(g) = +589.8kJ/mol. (c) Ei2 for Ca(g) = +1154kJ/mol. Heat...
-
Chemistry A one-electron atom is an atom with Z protons in the nucleus and one electron. For example, Z = 2 for helium and Z = 3 for lithium. Use our class discussion of the allowed radii and...
-
G-Bar Pastures is a 400-acre farm on the outskirts of the Kentucky Bluegrass, specializing in the boarding of broodmares and their foals. A recent economic downturn in the thoroughbred industry has...
-
Find the length of the curve. 12x = 4y 3 + 3y 1 , 1 y 3
-
What is the difference between the market value per share and the par value per share? AppendixLO1
-
During 2010, Minh Corporation had a net income of $144,000. Included on its income statement were depreciation expense of $16,000 and amortization expenses of $1,800. During the year, Accounts...
-
Required information The following data (in thousands of dollars) have been taken from the accounting records of Kartana Corporation for the year just ended 5910 588 Bales 30. materials inventory...
-
Air in a bicycle pump is compressed by pushing in the handle. The inner diameter of the pump is 3.0 cm and the pump is depressed 20. cm with a pressure of 2.00 atm. (a) How much work is done in the...
-
Which substance in each of the following pairs has the higher molar entropy? (Take the temperature to be 298 K unless otherwise specified.) (a) O 2 (g) or O 3 (g); (b) CH 2 Br 2 (g) or CH 4 (g); (c)...
-
Discuss the pros and cons of telephone interviews that take place during dinner time in the early evening?
-
Jennifer purchased stock at $50 per share with a 75% initial margin requirement and a maintenance margin of 35%. How much equity per share must Jennifer contribute when the stock falls to $15 per...
-
Thinking about your present job and your "inventory"of leadership traits and characteristics, where are your strengths and weaknesses as a leader?Is being a leader desirable? If yes, what motivates...
-
You are facing a complex decision with several courses of possible action and probabilities associated with them. The current decision tree, based on the best possible estimates of probabilities and...
-
1. In what ways has Marriot proven an industry leader in the context of entrepreneurship in the hospitality industry. 2. What are the author's metrics of measuring entrepreneurial activity, and do...
-
Suppose you want to model the relationship between the interest rate, the economic growth rate and the inflation rate. what would be first model to fit explain.
-
A phenotypically normal woman with an abnormally long chromo-some 13 (and a normal homolog of chromosome 13) marries a phe-notypically normal man with an abnormally short chromosome 11 (and a normal...
-
Find the intercepts and then graph the line. (a) 2x - 3y = 6 (b) 10 - 5x = 2y
-
The Ostwald process for the commercial production of nitric acid involves three steps: 2NO(g) + O2(g) 2NO2(g) 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) a. Calculate ÎHo, ÎSo, ÎGo, and K (at...
-
Consider the following reaction at 800. K: N2(g) + 3F2(g) 2NF3(g) An equilibrium mixture contains the following partial pressures: PN2 = 0.021 atm, PF2 = 0.063 atm, and PNF3 = 0.48 atm. Calculate Go...
-
Consider the following reaction at 298 K: 2SO2(g) + O2(g) 2SO3(g) An equilibrium mixture contains O2(g) and SO3(g) at partial pressures of 0.50 atm and 2.0 atm, respectively. Using data from...
-
Series of Compound Interest Techniques The following are several situations involving compound interest. Required: Using the appropriate table, solve each of the following: ( Click here to access the...
-
If Clark Kelly has recognized gain on an exchange of like-kind property held for investment use, where does Clark report the gain? First on Form 8824, then carried to Schedule D. First on Form 8824,...
-
An investor put 40% of her money in Stock A and 60% in Stock B. Stock A has a beta of 1.2 and Stock B has a beta of 1.6. If the risk-free rate is 5% and the expected return on the market is 12%,...

Study smarter with the SolutionInn App


