Consider a 0.60-M solution of HC 3 H 5 O 3 , lactic acid (Ka = 1.4
Question:
Consider a 0.60-M solution of HC3H5O3, lactic acid (Ka = 1.4 × 1024).
a. Which of the following are major species in the solution?
i. HC3H5O3
ii. C3H5O3
iii. H+
iv. H2O
v. OH-
b. Complete the following ICE table in terms of x, the amount (mol/L) of lactic acid that dissociates to reach equilibrium.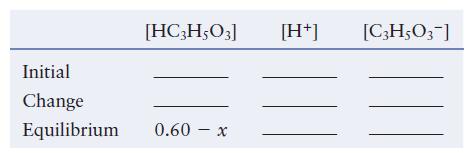
c. What is the equilibrium concentration for C3H5O3-?
d. Calculate the pH of the solution.
Transcribed Image Text:
Initial Change Equilibrium [HC3H5O3] 0.60 x - [H+] [C3H5O3-]
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 85% (7 reviews)
a The major species from above in...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Which of- the following are electron-deficient compounds? Explain. (a) (b) CH3 CH H C CHy H3C CH,
-
Which of the following are Section 1231 assets? Explain. Assume all the items have been held long-term. a. Machinery used in the business b. Personal home c. Factory building d. Land held as an...
-
Which of the following are private goods and might, therefore, be provided in socially optimal amounts by private profit-maximizers? Which are public goods and should, therefore, be provided by the...
-
You are the CEO of Green Paper Inc., a producer of high-end printing paper with an emphasis on environmentally friendly "green" production methods. One of your employees has proposed a significant...
-
Prove that the quantile function F1 of a general random variable X has the following three properties that are analogous to properties of the c.d.f.: a. F1 is a nondecreasing function of p for 0 < p...
-
When methyl ketones are treated with a halogen in the presence of base, the three hydrogen atoms on the methyl carbon are replaced to give a CX 3 -substituted ketone. This product is not stable under...
-
Why are comparative ratio analyses useful? AppendixLO1
-
A department of Alpha Co. incurred the following costs for the month of September. Variable costs, and the variable portion of mixed costs, are a function of the number of units of activity: Activity...
-
Samtech Manufacturing purchased land and building for $3 million. In addition to the purchase price, Samtech made the following expenditures in connection with the purchase of the land and building:...
-
Using an Excel spreadsheet, you will create a three-year ratio trend analysis from the financial statements data for Hershey corporation. Your spreadsheet must include actual formulas that calculate...
-
A 10.0-mL sample of an HCl solution has a pH of 2.000. What volume of water must be added to change the pH to 4.000?
-
A solution is prepared by adding 50.0 mL concentrated hydrochloric acid and 20.0 mL concentrated nitric acid to 300 mL water. More water is added until the final volume is 1.00 L. Calculate [H + ],...
-
Predict whether the following octahedral complexes are high spin or low spin, and given your prediction, give the number of unpaired electrons in each. Strategy Use a combination of the location in...
-
Recognition is a very important element of volunteer management. Do you know someone who has done amazing volunteer work for a good cause? Wouldn't it be nice to thank them with an award! Take a look...
-
What do Financial Planners do? Would you consider hiring a Financial Planner? How important are ethics when working with a financial planning professional? Explain the concept of return on...
-
Explain the specific perceptual errors you made of EACH of your teammates during the class exercise
-
Identify the company that makes the product a. Are they a large company or a small company? b. Are they a chain or a major corporation? c. Have they been around for decades or are they a new company?...
-
The Red Inn at Pismo is a 150-room hotel that caters mainly to business clients during the week and to tourists during the weekends and in the summer. Below is a table summarizing the average daily...
-
Why are many craters evident on the surface of the Moon but not on the surface of Earth?
-
Fill in each blank so that the resulting statement is true. 83 + 103 = ______ .
-
A compound contains only carbon, hydrogen, nitrogen, and oxygen. Combustion of 0.157 g of the compound produced 0.213 g of CO 2 and 0.0310 g of H 2 O. In another experiment, 0.103 g of the compound...
-
Maleic acid is an organic compound composed of 41.39% C, 3.47% H, and the rest oxygen. If 0.129 mole of maleic acid has a mass of 15.0 g, what are the empirical and molecular formulas of maleic acid?
-
Determine the molecular formula of a compound that contains 26.7% P, 12.1% N, and 61.2% Cl, and has a molar mass of 580 g/ mol.
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


