Question: Consider a cell based on the following half-reactions: a. Draw this cell under standard conditions, labeling the anode, the cathode, the direction of electron flow,
Consider a cell based on the following half-reactions:
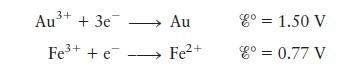
a. Draw this cell under standard conditions, labeling the anode, the cathode, the direction of electron flow, and the concentrations, as appropriate.
b. When enough \(\mathrm{NaCl}(s)\) is added to the compartment containing gold to make \(\left[\mathrm{Cl}^{-}ight]=0.10 \mathrm{M}\), the cell potential is observed to be \(0.31 \mathrm{~V}\). Assume that \(\mathrm{Au}^{3+}\) is reduced and that the reaction in the compartment containing gold is
\[\mathrm{Au}^{3+}(a q)+4 \mathrm{Cl}^{-}(a q) ightleftharpoons \mathrm{AuCl}_{4}^{-}(a q)\]
Calculate the value of \(K\) for this reaction at \(25^{\circ} \mathrm{C}\).
3+ Au + Au + 3e Fe+ + e Au Fe+ 8 = 1.50 V 8 = 0.77 V
Step by Step Solution
3.40 Rating (144 Votes )
There are 3 Steps involved in it
a To draw this cell under standard conditions you would include the two halfreactions provided 1 textAu3 3 texte ightarrow textAu with Ecirc 150 textV ... View full answer

Get step-by-step solutions from verified subject matter experts


