Question: the skills development exercises questions as well please i really dont get this ChemActivity 51 The Call Voltage 257 Information Table 1. Measured voltages for


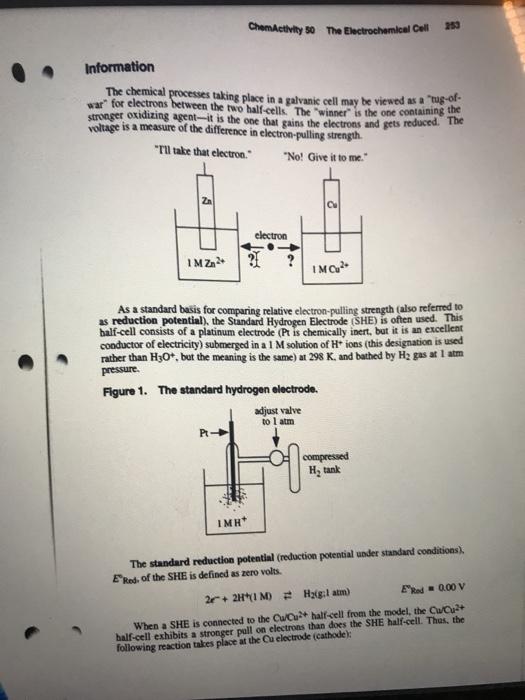
ChemActivity 51 The Call Voltage 257 Information Table 1. Measured voltages for some galvanic cells using standard electrodes (ali lons and soluble species at 1 M and all gases at 1 atm). Cathode Anode E (V) Cu/Cu2+ Zn/Zn2+ 1.10 Cu/Cu2+ SHE Bry/Br ZA/Zn2+ 1.85 Zn/Zn2+ K/K+ 2.16 Cl/C- Ag/Agt 0.56 Ag/Agt KK 3.72 0.34 Critical Thinking Questions 2. Determine the standard reduction potential, Ered, for each of the following half- reactions: Cl2 + 2 = 20 Br2 + 2 + 2 Br Ag+ + C Ag Cu2+ +20 Cu 2H+ + 2 H2 Zn2+ + 2 + Zn K+ + CK 3. What is the strongest oxidizing agent in the list in CTQ 2? What is the weakest oxidizing agent? 258 ChemActivity 51 The Cell Voltage 4. The stronger the oxidizing agent, the weaker the resulting reducing agent that is produced by the acquisition of electrons. In this case, what is the strongest reducing agent on the right-hand side of the list in CTQ 2? What is the weakest reducing agent? Skill Development Exercises Use a table of standard reduction potentials for the following exercises. 1. Assuming standard conditions, indicate which of the following is true: a) H2(g) can reduce Ag+(aq) b) H2(g) can reduce Ni2+(aq) c) Fe2+(aq) can reduce Cu2+(aq) d) H+(aq) can oxidize Mg(s) e) Pb2+(aq) can oxidize Ni(s) 2. A student places some Zn(s) powder in a beaker of 1 M nitric acid, and some Cu(s) powder in another beaker also containing 1 M nitric acid. In which, if any, of the beakers would you expect the solid to react and evolve hydrogen gas? Explain your reasoning 3. Find a reagent that can oxidize Br to Bry but cannot oxide Cl- to Ch2. 4. Indicate whether each of the following statements is true or false and explain your reasoning: a) The half-cell with the larger standard reduction potential is always the anode b) Whenever an oxidation half-reaction takes place, a reduction half-reaction must also take place. 5. You decide to construct a zinc/aluminum galvanic cell in which the electrodes are connected by a wire and the solutions are connected with a salt bridge. One electrode consists of an aluminum bar in a 1.0 M solution of aluminum(III) nitrate The other electrode consists of a zinc bar in a 1.0 M solution of zine(II) nitrate Zinc(II) has a more positive standard reduction potential than Al(III). Which electrode is the cathode and which is the anode? b) What is the direction of clectron flow? Which electrode is negative? Positive? What chemical reactions are occurring at each electrode? What is the overall chemical reaction? After a period of time, will the bar of zinc become heavier, lighter, or stay the same weight? Will the bar of aluminum become heavier, lighter, or stay the same weight? d) e) ChemActivity 50 The Electrochemical Coll Information The chemical processes taking place in a galvanis cell may be viewed as a tug-of- war for electrons between the two half-cells. The winner is the one containing the stronger oxidizing agent it is the one that gains the electrons and gets reduced. The voltage is a measure of the difference in electron-pulling strength. "Tu take that electron." "No! Give it to me Zn electron IM ZA IMG2 As a standard basis for comparing relative electron-pulling strength (also referred to as reduction potential), the Standard Hydrogen Electrode (SHE) is often used. This half-cell consists of a platinum electrode (Pt is chemically inert, but it is an excellent conductor of electricity) submerged in a 1 M solution of H+ ions (this designation is used rather than H30+, but the meaning is the same)at 298 K, and bathed by H2 gas at 1 atm pressure. Figure 1. The standard hydrogen electrode. adjust valve to atm PL - compressed H, tank IMH * The standard reduction potential (reduction potential under standard conditions) Ekod. of the SHE is defined as zero volts. 2.2H1M) Haiglatm) ER 0.00 V When a SHE is connected to the CW/Cu + half-cell from the model, the CWCuat half-cell exhibits a stronger pull on electrons than does the SHE half-cell. Thes, the following reaction takes place at the Cu electrode (Cathode Skill Development Questions 1. For the cell in the model, which is the stronger oxidizing agent-Zn2+ or Cu2+? 2. For the cell in the model, how much stronger (in terms of volts) is the stronger oxidizing agent? 3. Draw a galvanic cell consisting of a SHE and the copper electrode described above. Indicate a) the anode and the cathode, b) the direction of flow of the electrons in the wire, and c) which electrode is positive and which electrode is negative. Write down the half-reactions that are occurring at each electrode, and then write down the overall chemical process occurring in the cell. 4. When a standard Al(s)/Al3+ cell is connected to a SHE, the electrons are observed to flow in the direction of the SHE. The voltage is measured as 1.66 V. a) Identify the anode and the cathode in this system. b) Identify the positive and negative electrode. c) Give the half-reaction occurring in each half-cell, and then give the net chemical reaction for the cell. Keep in mind that the number of electrons being given up and being received must be the same d) What is the standard reduction potential for the Al(s)/A13+ half-cell? Explain your reasoning
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


