Consider the following reaction to produce methyl acetate: When this reaction is carried out with CH3OH containing
Question:
Consider the following reaction to produce methyl acetate: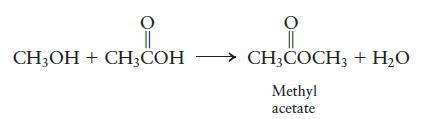
When this reaction is carried out with CH3OH containing radioactive oxygen-18, the water produced is not radioactive. Explain.
Transcribed Image Text:
CH3OH + CH3COH CH3COCH3 + H₂O Methyl acetate
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
My explanation is that the water that is formed during the react...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the following reaction to produce methyl acetate: When this reaction is carried out with CH3OH containing radioactive oxygen-18, the water produced is not radioactive. Explain. CH,OH CH...
-
Consider the reaction to produce the ester methyl acetate: When this reaction is carried our with CH3OH contain¬ing radioactive oxygen-18, the water produced does not contain oxygen-18. Explain...
-
When (S)-2-bromopropanoic acid [(S)-CH3CHBrCO2H] reacts with concentrated sodium hydroxide, the product formed (after acidification) is (R)-2-hydroxypropanoic acid [(R)-CH3CHOHCO2H, commonly known as...
-
A partially completed flowchart showing some of the major documents commonly used in the purchasing function of a merchandise business is presented below. Identify documents 1, 3, and4. Purchase Order
-
Use the frequency distribution from Try It Yourself 2 to construct a frequency polygon that represents the ages of the 50 most powerful women listed on page 39. Describe any patterns. 26, 31, 35, 37,...
-
Here is a collection of heights in centimeters together with the number of people in a group of that height (rounded to the nearest 5cm): (170,7), (175,9), (180,23), (185,17), (190,6), (195,1). How...
-
A ______ is a deliverable-oriented grouping of the work involved in a project that defines its total scope. a. scope statement b. WBS c. WBS dictionary d. work package LO.1
-
Sam Manuel has been employed on a full-time basis as an electrical engineer for the past three years. Prior to obtaining full-time employment, he was self-employed as an inventor of complex...
-
On August 1, 2021, Trico Technologies, an aeronautic electronics company, borrows $20.3 million cash to expand operations. The loan is made by FirstBanc Corp. under a short-term line of credit...
-
Imagine you are a new real estate salesperson looking for clients. Your broker tells you that client, S, is interested in selling her property. Your broker asks you to contact S and offer our...
-
Iodine-131 has a half-life of 8.0 days. How many days will it take for 174 g of 131 I to decay to 83 g of 131 I?
-
How could a radioactive nuclide be used to demonstrate that chemical equilibrium is a dynamic process?
-
Refer to the information for Raymond Company in Brief Exercise 6-34 and assume that the company uses the periodic inventory system. Refer to data exercise 6-34, Tyler Company has the following...
-
Briefly, discuss the use of survey research in exploratory, descriptive, explanatory, and evaluation studies. Using a criminal justice example select one type of research study and develop one...
-
Medical Helicopters In a study of helicopter usage and patient survival, results were obtained from 47,637 patients transported by helicopter and 111,874 patients transported by ground (based on data...
-
Woodland Hills Company reported income before taxes (pretax financial income) in its income statement of $60,000. Among the items included in the computation of pretax financial income were the...
-
cest Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this...
-
The activation energy for the gas phase decomposition of isobutyl bromide is 211 kJ. (CH3)2CHCH2 Br (CH3)2C=CH2+ HBr The rate constant at 676 K is 5.73 x 10-4 s. The rate constant will be 0.00647 s...
-
Solve each inequality. Give the answer using interval notation. -2(x - 1) - 12 < 2(x + 1)
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
Describe, in general, the structures of ionic solids. Com-pare and contrast the structures of sodium chloride and zinc sulfide. How many tetrahedral holes and octahedral holes are there per closest...
-
Assume the two-dimensional structure of an ionic com-pound MxAy is What is the empirical formula of this ionic compound?
-
Identify the most important types of interparticle forces present in the solids of each of the following substances. a. Ar b. HCl c. HF d. CaCl2 e. CH4 f. CO g. NaNO3 h. NH4Cl i. Teflon,...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...
-
Integrity Inc. can sell 20-year, $1,000 par value bonds paying semi-annual interests with a 10% coupon. The bonds can be sold for $1,050 each; flotation cost of $50 per bond will be incurred in this...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...

Study smarter with the SolutionInn App


